Genomic Insights into Pseudomonas protegens E1BL2 from Giant Jala Maize: A Novel Bioresource for Sustainable Agriculture and Efficient Management of Fungal Phytopathogens
- PMID: 39273455
- PMCID: PMC11395412
- DOI: 10.3390/ijms25179508
Genomic Insights into Pseudomonas protegens E1BL2 from Giant Jala Maize: A Novel Bioresource for Sustainable Agriculture and Efficient Management of Fungal Phytopathogens
Abstract
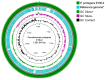
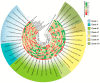
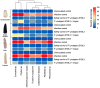
The relationships between plants and bacteria are essential in agroecosystems and bioinoculant development. The leaf endophytic Pseudomonas protegens E1BL2 was previously isolated from giant Jala maize, which is a native Zea mays landrace of Nayarit, Mexico. Using different Mexican maize landraces, this work evaluated the strain's plant growth promotion and biocontrol against eight phytopathogenic fungi in vitro and greenhouse conditions. Also, a plant field trial was conducted on irrigated fields using the hybrid maize Supremo. The grain productivity in this assay increased compared with the control treatment. The genome analysis of P. protegens E1BL2 showed putative genes involved in metabolite synthesis that facilitated its beneficial roles in plant health and environmental adaptation (bdhA, acoR, trpE, speE, potA); siderophores (ptaA, pchC); and extracellular enzymes relevant for PGPB mechanisms (cel3, chi14), protection against oxidative stress (hscA, htpG), nitrogen metabolism (nirD, nit1, hmpA), inductors of plant-induced systemic resistance (ISR) (flaA, flaG, rffA, rfaP), fungal biocontrol (phlD, prtD, prnD, hcnA-1), pest control (vgrG-1, higB-2, aprE, pslA, ppkA), and the establishment of plant-bacteria symbiosis (pgaA, pgaB, pgaC, exbD). Our findings suggest that P. protegens E1BL2 significantly promotes maize growth and offers biocontrol benefits, which highlights its potential as a bioinoculant.
Keywords: Pseudomonas protegens; antifungals; bacterial genome; biocontrol; maize endophyte; phytopathogen; promotion growth plant.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Wu T., Xu J., Xie W., Yao Z., Yang H., Sun C., Li X. Pseudomonas aeruginosa L10: A hydrocarbon-degrading, biosurfactant-producing, and plant-growth-promoting endophytic bacterium isolated from a reed (Phragmites australis) Front. Microbiol. 2018;9:1087. doi: 10.3389/fmicb.2018.01087. - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

