Moonlighting Crypto-Enzymes and Domains as Ancient and Versatile Signaling Devices
- PMID: 39273482
- PMCID: PMC11394779
- DOI: 10.3390/ijms25179535
Moonlighting Crypto-Enzymes and Domains as Ancient and Versatile Signaling Devices
Abstract
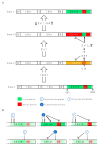
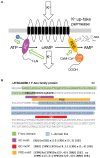
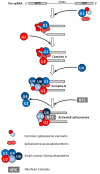
Increasing numbers of reports have revealed novel catalytically active cryptic guanylate cyclases (GCs) and adenylate cyclases (ACs) operating within complex proteins in prokaryotes and eukaryotes. Here we review the structural and functional aspects of some of these cyclases and provide examples that illustrate their roles in the regulation of the intramolecular functions of complex proteins, such as the phytosulfokine receptor (PSKR), and reassess their contribution to signal generation and tuning. Another multidomain protein, Arabidopsis thaliana K+ uptake permease (AtKUP5), also harbors multiple catalytically active sites including an N-terminal AC and C-terminal phosphodiesterase (PDE) with an abscisic acid-binding site. We argue that this architecture may enable the fine-tuning and/or sensing of K+ flux and integrate hormone responses to cAMP homeostasis. We also discuss how searches with motifs based on conserved amino acids in catalytic centers led to the discovery of GCs and ACs and propose how this approach can be applied to discover hitherto masked active sites in bacterial, fungal, and animal proteomes. Finally, we show that motif searches are a promising approach to discover ancient biological functions such as hormone or gas binding.
Keywords: H-NOX; abscisic acid (ABA); adenylate cyclase; crypto-domains; crypto-enzymes; guanylate cyclase; heme-proteins; phosphodiesterase; plant hormones; proteomes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Previero A., Coletti-Previero M.A., Galzigna L. Cryptic functions of enzymes in chemical catalysis. Monatshefte Für Chem./Chem. Mon. 1983;114:1059–1069. doi: 10.1007/BF00799030. - DOI
-
- Varfolomeev S.D. Catalytic centres of enzymes: Structural paradoxes, the phenomenon of structural unity and new reactions. Mendeleev Commun. 2004;14:185–188. doi: 10.1070/MC2004v014n05ABEH002020. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

