Fluoride Alters Gene Expression via Histone H3K27 Acetylation in Ameloblast-like LS8 Cells
- PMID: 39273544
- PMCID: PMC11395493
- DOI: 10.3390/ijms25179600
Fluoride Alters Gene Expression via Histone H3K27 Acetylation in Ameloblast-like LS8 Cells
Abstract
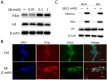
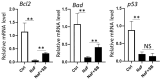
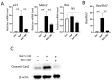
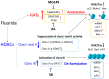
Excessive fluoride ingestion during tooth development can cause dental fluorosis. Previously, we reported that fluoride activates histone acetyltransferase (HAT) to acetylate p53, promoting fluoride toxicity in mouse ameloblast-like LS8 cells. However, the roles of HAT and histone acetylation status in fluoride-mediated gene expression remain unidentified. Here, we demonstrate that fluoride-mediated histone modification causes gene expression alterations in LS8 cells. LS8 cells were treated with or without fluoride followed by ChIP-Seq analysis of H3K27ac. Genes were identified by differential H3K27ac peaks within ±1 kb from transcription start sites. The levels of mRNA of identified genes were assessed using rea-time PCR (qPCR). Fluoride increased H3K27ac peaks associated with Bax, p21, and Mdm2 genes and upregulated their mRNA levels. Fluoride decreased H3K27ac peaks and p53, Bad, and Bcl2 had suppressed transcription. HAT inhibitors (Anacardic acid or MG149) suppressed fluoride-induced mRNA of p21 and Mdm2, while fluoride and the histone deacetylase (HDAC) inhibitor sodium butyrate increased Bad and Bcl2 expression above that of fluoride treatment alone. To our knowledge, this is the first study that demonstrates epigenetic regulation via fluoride treatment via H3 acetylation. Further investigation is required to elucidate epigenetic mechanisms of fluoride toxicity in enamel development.
Keywords: ChIP-Seq; HAT; HDAC; ameloblasts; amelogenesis; dental fluorosis; epigenetics; histone modification.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- U.S. Department of Health. Human Services Federal Panel on Community Water Fluoridation U.S Public Health Service Recommendation for Fluoride Concentration in Drinking Water for the Prevention of Dental Caries. Public Health Rep. 2015;130:318–331. doi: 10.1177/003335491513000408. - DOI - PMC - PubMed
-
- Asawa K., Singh A., Bhat N., Tak M., Shinde K., Jain S. Association of Temporomandibular Joint Signs & Symptoms with Dental Fluorosis & Skeletal Manifestations in Endemic Fluoride Areas of Dungarpur District, Rajasthan, India. J. Clin. Diagn. Res. 2015;9:ZC18–ZC21. doi: 10.7860/jcdr/2015/15807.6958. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

