Complement-Mediated Two-Step NETosis: Serum-Induced Complement Activation and Calcium Influx Generate NADPH Oxidase-Dependent NETs in Serum-Free Conditions
- PMID: 39273570
- PMCID: PMC11394910
- DOI: 10.3390/ijms25179625
Complement-Mediated Two-Step NETosis: Serum-Induced Complement Activation and Calcium Influx Generate NADPH Oxidase-Dependent NETs in Serum-Free Conditions
Abstract
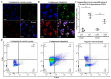
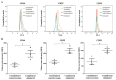
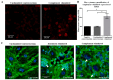
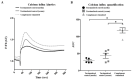
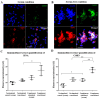
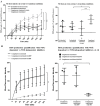
The complement system and neutrophils play crucial roles in innate immunity. Neutrophils release neutrophil extracellular traps (NETs), which are composed of decondensed DNA entangled with granular contents, as part of their innate immune function. Mechanisms governing complement-mediated NET formation remain unclear. In this study, we tested a two-step NETosis mechanism, as follows: classical complement-mediated neutrophil activation in serum and subsequent NET formation in serum-free conditions, using neutrophils from healthy donors, endothelial cells, and various assays (Fluo-4AM, DHR123, and SYTOX), along with flow cytometry and confocal microscopy. Our findings reveal that classical complement activation on neutrophils upregulated the membrane-anchored complement regulators CD46, CD55, and CD59. Additionally, complement activation increased CD11b on neutrophils, signifying activation and promoting their attachment to endothelial cells. Complement activation induced calcium influx and citrullination of histone 3 (CitH3) in neutrophils. However, CitH3 formation alone was insufficient for NET generation. Importantly, NET formation occurred only when neutrophils were in serum-free conditions. In such environments, neutrophils induced NADPH oxidase-dependent reactive oxygen species (ROS) production, leading to NET formation. Hence, we propose that complement-mediated NET formation involves a two-step process, as follows: complement deposition, neutrophil priming, calcium influx, CitH3 formation, and attachment to endothelial cells in serum. This is followed by NADPH-dependent ROS production and NET completion in serum-free conditions. Understanding this process may unveil treatment targets for pathologies involving complement activation and NET formation.
Keywords: NET formation; NETosis; P-selectin/CD11b; citrullinated histone 3; complement; neutrophils.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

