The Functions of SARS-CoV-2 Receptors in Diabetes-Related Severe COVID-19
- PMID: 39273582
- PMCID: PMC11394807
- DOI: 10.3390/ijms25179635
The Functions of SARS-CoV-2 Receptors in Diabetes-Related Severe COVID-19
Abstract
Angiotensin-converting enzyme 2 (ACE2) is considered a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor of high importance, but due to its non-ubiquitous expression, studies of other proteins that may participate in virus internalisation have been undertaken. To date, many alternative receptors have been discovered. Their functioning may provide an explanation for some of the events observed in severe COVID-19 that cannot be directly explained by the model in which ACE2 constitutes the central point of infection. Diabetes mellitus type 2 (T2D) can induce severe COVID-19 development. Although many mechanisms associated with ACE2 can lead to increased SARS-CoV-2 virulence in diabetes, proteins such as basigin (CD147), glucose-regulated protein 78 kDa (GRP78), cluster of differentiation 4 (CD4), transferrin receptor (TfR), integrins α5β1/αvβ3, or ACE2 co-receptors neuropilin 2 (NRP2), vimentin, and even syalilated gangliosides may also be responsible for worsening the COVID-19 course. On the other hand, some others may play protective roles. Understanding how diabetes-associated mechanisms can induce severe COVID-19 via modification of virus receptor functioning needs further extensive studies.
Keywords: ACE2; CD147; CD4; GRP78; NRP2; SARS-CoV-2; TfR; diabetes mellitus type 2; integrins; sialic acid; vimentin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


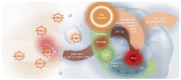
Similar articles
-
ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities.Elife. 2020 Nov 9;9:e61390. doi: 10.7554/eLife.61390. Elife. 2020. PMID: 33164751 Free PMC article. Review.
-
SARS-CoV-2 Entry: At the Crossroads of CD147 and ACE2.Cells. 2021 Jun 8;10(6):1434. doi: 10.3390/cells10061434. Cells. 2021. PMID: 34201214 Free PMC article.
-
Possible Involvement of Adipose Tissue in Patients With Older Age, Obesity, and Diabetes With SARS-CoV-2 Infection (COVID-19) via GRP78 (BIP/HSPA5): Significance of Hyperinsulinemia Management in COVID-19.Diabetes. 2021 Dec;70(12):2745-2755. doi: 10.2337/db20-1094. Epub 2021 Oct 6. Diabetes. 2021. PMID: 34615619 Free PMC article. Review.
-
The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: inflammasome and JaK inhibitors as potential promising therapies.Cell Mol Biol Lett. 2022 Feb 2;27(1):10. doi: 10.1186/s11658-022-00311-1. Cell Mol Biol Lett. 2022. PMID: 35109786 Free PMC article. Review.
-
Can SARS-CoV-2 Virus Use Multiple Receptors to Enter Host Cells?Int J Mol Sci. 2021 Jan 20;22(3):992. doi: 10.3390/ijms22030992. Int J Mol Sci. 2021. PMID: 33498183 Free PMC article. Review.
Cited by
-
Effect of Ficus pumila L. on Improving Insulin Secretory Capacity and Resistance in Elderly Patients Aged 80 Years Old or Older Who Develop Diabetes After COVID-19 Infection.Nutrients. 2025 Jan 15;17(2):290. doi: 10.3390/nu17020290. Nutrients. 2025. PMID: 39861420 Free PMC article.
-
Myeloid-Derived Suppressor Cells (MDSCs) and Obesity-Induced Inflammation in Type 2 Diabetes.Diagnostics (Basel). 2024 Nov 1;14(21):2453. doi: 10.3390/diagnostics14212453. Diagnostics (Basel). 2024. PMID: 39518420 Free PMC article. Review.
References
-
- Floyd J.S., Walker R.L., Kuntz J.L., Shortreed S.M., Fortmann S.P., Bayliss E.A., Harrington L.B., Fuller S., Albertson-Junkans L.H., Powers J.D., et al. Association Between Diabetes Severity and Risks of COVID-19 Infection and Outcomes. J. Gen. Intern. Med. 2023;38:1484–1492. doi: 10.1007/s11606-023-08076-9. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

