The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens
- PMID: 39273619
- PMCID: PMC11395145
- DOI: 10.3390/ijms25179672
The Archetypal Gamma-Core Motif of Antimicrobial Cys-Rich Peptides Inhibits H+-ATPases in Target Pathogens
Abstract
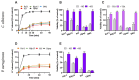
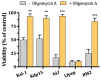
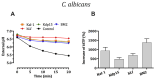
Human lactoferrin (hLf) is an innate host defense protein that inhibits microbial H+-ATPases. This protein includes an ancestral structural motif (i.e., γ-core motif) intimately associated with the antimicrobial activity of many natural Cys-rich peptides. Peptides containing a complete γ-core motif from hLf or other phylogenetically diverse antimicrobial peptides (i.e., afnA, SolyC, PA1b, PvD1, thanatin) showed microbicidal activity with similar features to those previously reported for hLf and defensins. Common mechanistic characteristics included (1) cell death independent of plasma membrane (PM) lysis, (2) loss of intracellular K+ (mediated by Tok1p K+ channels in yeast), (3) inhibition of microbicidal activity by high extracellular K+, (4) influence of cellular respiration on microbicidal activity, (5) involvement of mitochondrial ATP synthase in yeast cell death processes, and (6) increment of intracellular ATP. Similar features were also observed with the BM2 peptide, a fungal PM H+-ATPase inhibitor. Collectively, these findings suggest host defense peptides containing a homologous γ-core motif inhibit PM H+-ATPases. Based on this discovery, we propose that the γ-core motif is an archetypal effector involved in the inhibition of PM H+-ATPases across kingdoms of life and contributes to the in vitro microbicidal activity of Cys-rich antimicrobial peptides.
Keywords: ATPase inhibitor; H+-ATPase; antimicrobial mechanism of action; antimicrobial motif; antimicrobial peptide; gamma-core motif; host defense; innate immunity; lactoferrin.
Conflict of interest statement
The authors report no conflicts of interest.
Figures

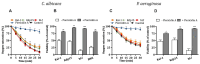

References
-
- Dalmastri C., Valenti P., Visca P., Vittorioso P., Orsi N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica. 1988;11:225–230. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

