Functional Analysis of RE1 Silencing Transcription Factor as a Putative Tumor Suppressor in Human Endometrial Cancer
- PMID: 39273639
- PMCID: PMC11395688
- DOI: 10.3390/ijms25179693
Functional Analysis of RE1 Silencing Transcription Factor as a Putative Tumor Suppressor in Human Endometrial Cancer
Abstract
Uterine cancer is the most common gynecologic malignancy in the United States, with endometrioid endometrial adenocarcinoma (EC) being the most common histologic sub-type. Considering the molecular classifications of EC, efforts have been made to identify additional biomarkers that can assist in diagnosis, prognosis, and individualized therapy. We sought to explore the relationship of Repressor Element 1 (RE1) silencing transcription factor (REST), which downregulates neuronal genes in non-neuronal tissue, along with matrix metalloproteinase-24 (MMP24) and EC. We analyzed the expression of REST and MMP24 in 31 cases of endometrial cancer and 16 controls. We then explored the baseline expression of REST and MMP24 in two EC cell lines (Ishikawa and HEC-1-A) compared to a benign cell line (t-HESC) and subsequently evaluated proliferation, migration, and invasion in the setting of loss of REST gene expression. REST and MMP24 expression were significantly lower in human EC samples compared to control samples. REST was highly expressed in EC cell lines, but decreasing REST gene expression increased proliferation (FC: 1.13X, p < 0.0001), migration (1.72X, p < 0.0001), and invasion (FC: 7.77X, p < 0.05) in Ishikawa cells, which are hallmarks of cancer progression and metastasis. These findings elicit a potential role for REST as a putative tumor suppressor in EC.
Keywords: MMP24; REST; endometrial cancer; endometrioid sub-type; gene expression; invasion; migration; proliferation; protein expression.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



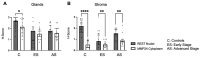






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

