Anti-Inflammatory and Anti-(Lymph)angiogenic Properties of an ABCB5+ Limbal Mesenchymal Stem Cell Population
- PMID: 39273646
- PMCID: PMC11395824
- DOI: 10.3390/ijms25179702
Anti-Inflammatory and Anti-(Lymph)angiogenic Properties of an ABCB5+ Limbal Mesenchymal Stem Cell Population
Abstract
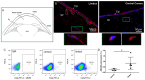
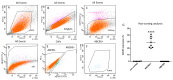
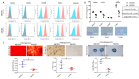
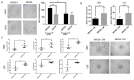
Corneal transparency and avascularity are essential for vision. The avascular cornea transitions into the vascularized conjunctiva at the limbus. Here, we explore a limbal stromal cell sub-population that expresses ABCB5 and has mesenchymal stem cell characteristics. Human primary corneal stromal cells were enriched for ABCB5 by using FACS sorting. ABCB5+ cells expressed the MSC markers CD90, CD73, and CD105. ABCB5+ but not ABCB5- cells from the same donor displayed evidence of pluripotency with a significantly higher colony-forming efficiency and the ability of trilineage differentiation (osteogenic, adipogenic, and chondrogenic). The ABCB5+ cell secretome demonstrated lower levels of the pro-inflammatory protein MIF (macrophage migration inhibitory factor) as well as of the pro-(lymph)angiogenic growth factors VEGFA and VEGFC, which correlated with reduced proliferation of Jurkat cells co-cultured with ABCB5+ cells and decreased proliferation of blood and lymphatic endothelial cells cultured in ABCB5+ cell-conditioned media. These data support the hypothesis that ABCB5+ limbal stromal cells are a putative MSC population with potential anti-inflammatory and anti-(lymph)angiogenic effects. The therapeutic modulation of ABCB5+ limbal stromal cells may prevent cornea neovascularization and inflammation and, if transplanted to other sites in the body, provide similar protective properties to other tissues.
Keywords: (lymph)angiogenesis; ABCB5; cornea; mesenchymal stem cells.
Conflict of interest statement
C.G.: CEO of RHEACELL GmbH & Co. KG. M.A.K.: CSO of RHEACELL GmbH & Co. KG. M.H.F., B.R.K., and N.Y.F. are inventors or co-inventors of US and international patents assigned to Brigham and Women’s Hospital, Boston Children’s Hospital, the Massachusetts Eye and Ear Infirmary, and/or the VA Boston Healthcare System, Boston, MA, licensed to RHEACELL GmbH & Co. KG (Heidelberg, Germany). M.H.F. serves as a scientific advisor to and holds stock in RHEACELL GmbH & Co. KG.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

