Antimicrobial Activity of Chalcones with a Chlorine Atom and Their Glycosides
- PMID: 39273666
- PMCID: PMC11395246
- DOI: 10.3390/ijms25179718
Antimicrobial Activity of Chalcones with a Chlorine Atom and Their Glycosides
Abstract
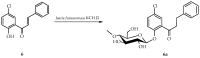
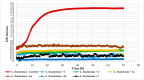
Chalcones, secondary plant metabolites, exhibit various biological properties. The introduction of a chlorine and a glucosyl substituent to the chalcone could enhance its bioactivity and bioavailability. Such compounds can be obtained through a combination of chemical and biotechnological methods. Therefore, 4-chloro-2'-hydroxychalcone and 5'-chloro-2'-hydroxychalcone were obtained by synthesis and then glycosylated in two filamentous fungi strains cultures, i.e., Isaria fumosorosea KCH J2 and Beauveria bassiana KCH J1.5. The main site of the glycosylation of both compounds by I. fumosorosea KCH J2 was C-2' and C-3 when the second strain was utilized. The pharmacokinetics of these compounds were predicted using chemoinformatics tools. Furthermore, antimicrobial activity tests were performed. Compounds significantly inhibited the growth of the bacteria strains Escherichia coli 10536, Staphylococcus aureus DSM 799, and yeast Candida albicans DSM 1386. Nevertheless, the bacterial strain Pseudomonas aeruginosa DSM 939 exhibited significant resistance to their effects. The growth of lactic acid bacteria strain Lactococcus acidophilus KBiMZ 01 bacteria was moderately inhibited, but strains Lactococcus rhamnosus GG and Streptococcus thermophilus KBM-1 were completely inhibited. In summary, chalcones substituted with a chlorine demonstrated greater efficacy in inhibiting the microbial strains under examination compared to 2'-hydroxychalcone, while aglycones and their glycosides exhibited similar effectiveness.
Keywords: Beauveria bassiana; Isaria fumosorosea; antimicrobial activity; biotransformations; chalcones with a chlorine atom; glycosylated dihydrochalcones.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
















References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

