Distinct Variations in Gene Expression and Cell Composition across Lichen Planus Subtypes
- PMID: 39273670
- PMCID: PMC11396712
- DOI: 10.3390/ijms25179720
Distinct Variations in Gene Expression and Cell Composition across Lichen Planus Subtypes
Abstract
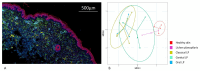
Lichen planus (LP) is a highly prevalent inflammatory skin disease. While various clinical subtypes have been defined, detailed comparisons of these variants are lacking. This study aimed to elucidate differences in gene expression and cellular composition across LP subtypes. Lesional skin biopsies from 28 LP patients (classical, oral, genital, and lichen planopilaris) and seven non-diseased skin controls (NDC) were analyzed. Gene expression profiling of 730 inflammation-related genes was conducted using NanoString. Immune cell compositions were assessed by multiplex immunohistochemistry. Gene expression profiles revealed unique inflammatory signatures for each LP subtype. Lichen planopilaris exhibited the most divergence, with downregulated gene expression and upregulation of complement pathway genes (C5-7), along with elevated M2 macrophages. Oral and genital LP demonstrated similar profiles with strong upregulation of TNF-related and Toll-like receptor-associated genes. Oral LP showed the highest upregulation of cytotoxicity-associated genes, as well as high numbers of CD8+ IL-17A+ (Tc17) cells (8.02%). Interferon gene signatures were strongly upregulated in oral and classical LP. The study highlights distinct differences in inflammatory gene expression and cell composition across LP subtypes, emphasizing the need for tailored therapeutic approaches.
Keywords: NanoString; immune cell infiltrate; lichen planus; lichen planus subtypes; multiplex immunohistochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Significant reduction in the expression of interleukins-17A, 22 and 23A, forkhead box p3 and interferon gamma delineates lichen planus pigmentosus from lichen planus.Arch Dermatol Res. 2019 Sep;311(7):519-527. doi: 10.1007/s00403-019-01926-9. Epub 2019 May 14. Arch Dermatol Res. 2019. PMID: 31089878
-
Distinct Immunological Features Compared to Lichen Planus and Oral Lichen Planus.J Inflamm Res. 2025 Mar 18;18:4037-4056. doi: 10.2147/JIR.S506313. eCollection 2025. J Inflamm Res. 2025. PMID: 40125076 Free PMC article.
-
Therapeutic Targeting of Th17/Tc17 Cells Leads to Clinical Improvement of Lichen Planus.Front Immunol. 2019 Jul 31;10:1808. doi: 10.3389/fimmu.2019.01808. eCollection 2019. Front Immunol. 2019. PMID: 31417572 Free PMC article.
-
Ocular involvement and complications of lichen planus, lichen planus pigmentosus, and lichen planopilaris: A comprehensive review.Dermatol Ther. 2021 Nov;34(6):e15137. doi: 10.1111/dth.15137. Epub 2021 Sep 25. Dermatol Ther. 2021. PMID: 34541780 Review.
-
JAK inhibitors in lichen planus: a review of pathogenesis and treatments.J Dermatolog Treat. 2022 Dec;33(8):3098-3103. doi: 10.1080/09546634.2022.2116926. Epub 2022 Aug 29. J Dermatolog Treat. 2022. PMID: 35997540 Review.
References
-
- Ioannides D., Vakirlis E., Kemeny L., Marinovic B., Massone C., Murphy R., Nast A., Ronnevig J., Ruzicka T., Cooper S., et al. European S1 guidelines on the management of lichen planus: A cooperation of the European Dermatology Forum with the European Academy of Dermatology and Venereology. J. Eur. Acad. Dermatol. Venereol. 2020;34:1403–1414. doi: 10.1111/jdv.16464. - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

