Neutrophil Biomarkers Can Predict Cardiotoxicity of Anthracyclines in Breast Cancer
- PMID: 39273682
- PMCID: PMC11395913
- DOI: 10.3390/ijms25179735
Neutrophil Biomarkers Can Predict Cardiotoxicity of Anthracyclines in Breast Cancer
Abstract
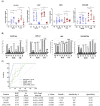
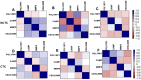
Doxorubicin (DOX), a commonly used anticancer agent, causes cardiotoxicity that begins with the first dose and may progress to heart failure years after treatment. An inflammatory response associated with neutrophil recruitment has been recognized as a mechanism of DOX-induced cardiotoxicity. This study aimed to validate mRNA expression of the previously identified biomarkers of DOX-induced cardiotoxicity, PGLYRP1, CAMP, MMP9, and CEACAM8, and to assay their protein expression in the peripheral blood of breast cancer patients. Blood samples from 40 breast cancer patients treated with DOX-based chemotherapy were collected before and after the first chemotherapy cycle and > 2 years after treatment. The protein and gene expression of PGLYRP1/Tag7, CAMP/LL37, MMP9/gelatinase B, and CEACAM8/CD66b were determined using ELISA and reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Receiver operating characteristic (ROC) curve analysis was used to determine the diagnostic value of each candidate biomarker. Patients with cardiotoxicity (n = 20) had significantly elevated levels of PGLYRP1, CAMP, MMP9, and CEACAM8 at baseline, after the first dose of DOX-based chemotherapy, and at > 2 years after treatment relative to patients without cardiotoxicity (n = 20). The first dose of DOX induced significantly higher levels of all examined biomarkers in both groups of patients. At > 2 years post treatment, the levels of all but MMP9 dropped below the baseline. There was a good correlation between the expression of mRNA and the target proteins. We demonstrate that circulating levels of PGLYRP1, CAMP, MMP9, and CEACAM8 can predict the cardiotoxicity of DOX. This novel finding may be of value in the early identification of patients at risk for cardiotoxicity.
Keywords: ELISA; RT-qPCR; anthracyclines; cardiotoxicity; innate immunity; neutrophils.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Kaboré E.G., Macdonald C., Kaboré A., Didier R., Arveux P., Meda N., Boutron-Ruault M.C., Guenancia C. Risk Prediction Models for Cardiotoxicity of Chemotherapy Among Patients with Breast Cancer: A Systematic Review. JAMA Netw. Open. 2023;6:e230569. doi: 10.1001/jamanetworkopen.2023.0569. - DOI - PMC - PubMed
-
- Dulf P.L., Mocan M., Coadă C.A., Dulf D.V., Moldovan R., Baldea I., Farcas A.D., Blendea D., Filip A.G. Doxorubicin-induced acute cardiotoxicity is associated with increased oxidative stress, autophagy, and inflammation in a murine model. Naunyn. Schmiedebergs. Arch. Pharmacol. 2023;396:1105–1115. doi: 10.1007/s00210-023-02382-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

