L-Fucose-Rich Sulfated Glycans from Edible Brown Seaweed: A Promising Functional Food for Obesity and Energy Expenditure Improvement
- PMID: 39273687
- PMCID: PMC11395595
- DOI: 10.3390/ijms25179738
L-Fucose-Rich Sulfated Glycans from Edible Brown Seaweed: A Promising Functional Food for Obesity and Energy Expenditure Improvement
Abstract
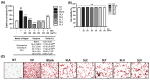
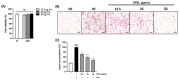
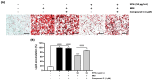
The global obesity epidemic, exacerbated by the sedentary lifestyle fostered by the COVID-19 pandemic, presents a growing socioeconomic burden due to decreased physical activity and increased morbidity. Current obesity treatments show promise, but they often come with expensive medications, frequent injections, and potential side effects, with limited success in improving obesity through increased energy expenditure. This study explores the potential of a refined sulfated polysaccharide (SPSL), derived from the brown seaweed Scytosiphon lomentaria (SL), as a safe and effective anti-obesity treatment by promoting energy expenditure. Chemical characterization revealed that SPSL, rich in sulfate and L-fucose content, comprises nine distinct sulfated glycan structures. In vitro analysis demonstrated potent anti-lipogenic properties in adipocytes, mediated by the downregulation of key adipogenic modulators, including 5' adenosine monophosphate-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor γ (PPARγ) pathways. Inhibiting AMPK attenuated the anti-adipogenic effects of SPSL, confirming its involvement in the mechanism of action. Furthermore, in vivo studies using zebrafish models showed that SPSL increased energy expenditure and reduced lipid accumulation. These findings collectively highlight the therapeutic potential of SPSL as a functional food ingredient for mitigating obesity-related metabolic dysregulation by promoting energy expenditure. Further mechanistic and preclinical investigations are warranted to fully elucidate its mode of action and evaluate its efficacy in obesity management, potentially offering a novel, natural therapeutic avenue for this global health concern.
Keywords: AMPK; Scytosiphon lomentaria; brown seaweed; energy expenditure; fucoidan; metabolic disorders; sulfated glycans; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Puska P., Nishida C., Porter D., World Health Organization . Obesity and Overweight. World Health Organization; Geneva, Switzerland: 2003. pp. 1–2.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

