Block Copolymers of Poly(N-Vinyl Pyrrolidone) and Poly(Vinyl Esters) Bearing n-alkyl Side Groups via Reversible Addition-Fragmentation Chain-Transfer Polymerization: Synthesis, Characterization, and Thermal Properties
- PMID: 39274080
- PMCID: PMC11398064
- DOI: 10.3390/polym16172447
Block Copolymers of Poly(N-Vinyl Pyrrolidone) and Poly(Vinyl Esters) Bearing n-alkyl Side Groups via Reversible Addition-Fragmentation Chain-Transfer Polymerization: Synthesis, Characterization, and Thermal Properties
Abstract
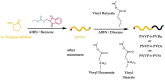
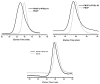
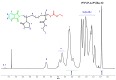
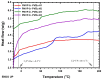
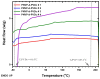
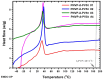
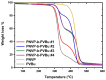
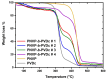
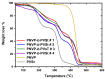
Amphiphilic block copolymers of N-vinyl pyrrolidone (NVP) and various vinyl esters (VEs), PNVP-b-PVEs, namely vinyl butyrate (VBu), vinyl decanoate (VDc), and vinyl stearate (VSt), were synthesized through RAFT polymerization techniques. The sequential addition of the monomers methodology was employed starting from the polymerization of NVP followed by the polymerization of the Ves' monomer. The polymerization of NVP was conducted at 60 °C in benzene solution using AIBN as the initiator and O-ethyl S-(phthalimidylmethyl) xanthate as the CTA. The resulting PNVP macro-CTA was further applied for the polymerization of the vinyl ester in dioxane solution at 80 °C using, again, AIBN as the initiator. The block copolymers were characterized through size-exclusion chromatography (SEC) and NMR spectroscopy. The thermal behavior of the copolymers was studied by Differential Scanning Calorimetry (DSC), whereas their thermal stability via Thermogravimetric Analysis (TGA) and Differential Thermogravimetry (DTG).
Keywords: RAFT polymerization; block copolymers; poly(N-vinyl pyrrolidone) (NVP); poly(vinyl esters) (VEs); thermal analysis; thermal stability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Synthesis and Micellization Behavior of Amphiphilic Block Copolymers of Poly(N-vinyl Pyrrolidone) and Poly(Benzyl Methacrylate): Block versus Statistical Copolymers.Polymers (Basel). 2023 May 8;15(9):2225. doi: 10.3390/polym15092225. Polymers (Basel). 2023. PMID: 37177372 Free PMC article.
-
Statistical Copolymers of N-Vinylpyrrolidone and 2-Chloroethyl Vinyl Ether via Radical RAFT Polymerization: Monomer Reactivity Ratios, Thermal Properties, and Kinetics of Thermal Decomposition of the Statistical Copolymers.Polymers (Basel). 2023 Apr 21;15(8):1970. doi: 10.3390/polym15081970. Polymers (Basel). 2023. PMID: 37112117 Free PMC article.
-
Synthesis, Characterization, and Self-Assembly Behavior of Block Copolymers of N-Vinyl Pyrrolidone with n-Alkyl Methacrylates.Polymers (Basel). 2025 Apr 21;17(8):1122. doi: 10.3390/polym17081122. Polymers (Basel). 2025. PMID: 40284387 Free PMC article.
-
Recent Advances in the Synthesis of Complex Macromolecular Architectures Based on Poly(N-vinyl pyrrolidone) and the RAFT Polymerization Technique.Polymers (Basel). 2022 Feb 11;14(4):701. doi: 10.3390/polym14040701. Polymers (Basel). 2022. PMID: 35215614 Free PMC article. Review.
-
Amino-acid-based block copolymers by RAFT polymerization.Macromol Rapid Commun. 2012 Jul 13;33(13):1090-107. doi: 10.1002/marc.201100887. Epub 2012 Apr 17. Macromol Rapid Commun. 2012. PMID: 22508409 Review.
References
-
- Rinno H. Ullmann’s Encyclopedia of Industrial Chemistry. Volume 28. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2000. Poly(vinyl esters) pp. 469–479.
-
- Mark H.F., Bikales N., Overberger C.G., Menges G., Kroschwitz J.I. Encyclopedia of Polymer Science and Engineering. 2nd ed. Volume 17. Wiley; New York, NY, USA: 1989. p. 420.
-
- Ahmad H., Hasan M.K., Miah M.A.J., Ali A.M.I., Tauer K. Solvent effect on the emulsion copolymerization of MMA and LMA in aqueous media. Polymer. 2011;52:3925–3932. doi: 10.1016/j.polymer.2011.07.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

