Coaxial Bioprinting of Enzymatically Crosslinkable Hyaluronic Acid-Tyramine Bioinks for Tissue Regeneration
- PMID: 39274103
- PMCID: PMC11398246
- DOI: 10.3390/polym16172470
Coaxial Bioprinting of Enzymatically Crosslinkable Hyaluronic Acid-Tyramine Bioinks for Tissue Regeneration
Abstract
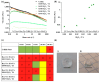
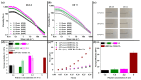
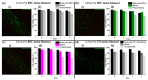
Three-dimensional (3D) bioprinting has emerged as an important technique for fabricating tissue constructs with precise structural and compositional control. However, developing suitable bioinks with biocompatible crosslinking mechanisms remains a significant challenge. This study investigates extrusion-based bioprinting (EBB) using uniaxial or coaxial nozzles with enzymatic crosslinking (EC) to produce 3D tissue constructs in vitro. Initially, low-molecular-weight dextran-tyramine and hyaluronic acid-tyramine (LMW Dex-TA/HA-TA) bioink prepolymers were evaluated. Enzymatically pre-crosslinking these prepolymers, achieved by the addition of horseradish peroxidase and hydrogen peroxide, produced viscous polymer solutions. However, this approach resulted in inconsistent bioprinting outcomes (uniaxial) due to inhomogeneous crosslinking, leading to irreproducible properties and suboptimal shear recovery behavior of the hydrogel inks. To address these challenges, we explored a one-step coaxial bioprinting system consisting of enzymatically crosslinkable high-molecular-weight hyaluronic acid-tyramine conjugates (HMW HA-TA) mixed with horseradish peroxidase (HRP) in the inner core and a mixture of Pluronic F127 and hydrogen peroxide in the outer shell. This configuration resulted in nearly instantaneous gelation by diffusion of the hydrogen peroxide into the core. Stable hydrogel fibers with desirable properties, including appropriate swelling ratios and controlled degradation rates, were obtained. The optimized bioink and printing parameters included 1.3% w/v HMW HA-TA and 5.5 U/mL HRP (bioink, inner core), and 27.5% w/v Pluronic F127 and 0.1% H2O2 (sacrificial ink, outer shell). Additionally, optimal pressures for the inner core and outer shell were 45 and 80 kPa, combined with a printing speed of 300 mm/min and a bed temperature of 30 °C. The extruded HMW HA-TA core filaments, containing bovine primary chondrocytes (BPCs) or 3T3 fibroblasts (3T3 Fs), exhibited good cell viabilities and were successfully cultured for up to seven days. This study serves as a proof-of-concept for the one-step generation of core filaments using a rapidly gelling bioink with an enzymatic crosslinking mechanism, and a coaxial bioprinter nozzle system. The results demonstrate significant potential for developing designed, printed, and organized 3D tissue fiber constructs.
Keywords: bioink; coaxial bioprinting; enzymatic crosslinking; hyaluronic acid-tyramine conjugates; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

