Clinical and Hemodynamic Outcomes with Enalapril Orodispersible Minitablets in Young Children with Heart Failure Due to Congenital Heart Disease
- PMID: 39274188
- PMCID: PMC11396157
- DOI: 10.3390/jcm13174976
Clinical and Hemodynamic Outcomes with Enalapril Orodispersible Minitablets in Young Children with Heart Failure Due to Congenital Heart Disease
Abstract
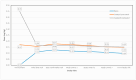
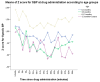
Background: The angiotensin-converting enzyme inhibitor (ACEI) enalapril is often administered to infants and young children with heart failure (HF) in various dosing regimens and formulations not adapted for their age. Methods: This prospective, two-center, open-label 8-week study evaluated an age-appropriate formulation of orodispersible minitablets (ODMTs) of enalapril (0.25 mg and 1 mg) in children aged 0 to 6 years with HF due to congenital heart disease. An age/weight-based dosing schedule was followed. Measures of echocardiographic parameters, blood pressure, heart rate, modified Ross score, and biochemistry were obtained over the 8-week period. The following two groups were assessed: ACEI-naïve and ACEI-pretreated patients. Results: In total, 53 children (age range of 0.05 to 4.8 years) were enrolled and 29 were ACEI-naïve. The average enalapril dose was 0.098 mg/kg (0.06-0.17 mg/kg) in the naïve group and 0.15 mg/kg (0.07-0.3 mg/kg) in pretreated patients. After 8 weeks, the modified Ross score and left ventricular diastolic dimension (LVD) z-score showed a significant decrease in both groups (p < 0.005). During 8 weeks follow-up, there were no difference in the z-scores for the systolic blood pressure (p = 0.071) or heart rate (p = 0.146). Conclusions: Pediatric patients treated with ODMTs of enalapril for 8 weeks had favorable improvements in LVD and HF symptoms.
Keywords: ACEIs; congenital heart disease; enalapril; heart failure; orodispersible minitablets; pediatric cardiology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Acceptability and Palatability of Novel Orodispersible Minitablets of Enalapril in Children up to the Age of 6 with Heart Failure.J Clin Med. 2025 Jan 30;14(3):915. doi: 10.3390/jcm14030915. J Clin Med. 2025. PMID: 39941586 Free PMC article.
-
Enalapril and Enalaprilat Pharmacokinetics in Children with Heart Failure Due to Dilated Cardiomyopathy and Congestive Heart Failure after Administration of an Orodispersible Enalapril Minitablet (LENA-Studies).Pharmaceutics. 2022 May 30;14(6):1163. doi: 10.3390/pharmaceutics14061163. Pharmaceutics. 2022. PMID: 35745735 Free PMC article.
-
Orodispersible minitablets of enalapril for use in children with heart failure (LENA): Rationale and protocol for a multicentre pharmacokinetic bridging study and follow-up safety study.Contemp Clin Trials Commun. 2019 Jun 8;15:100393. doi: 10.1016/j.conctc.2019.100393. eCollection 2019 Sep. Contemp Clin Trials Commun. 2019. PMID: 31249901 Free PMC article.
-
Angiotensin receptor/neprilysin inhibitor-a breakthrough in chronic heart failure therapy: summary of subanalysis on PARADIGM-HF trial findings.Heart Fail Rev. 2020 May;25(3):393-402. doi: 10.1007/s10741-019-09879-x. Heart Fail Rev. 2020. PMID: 31713710 Free PMC article. Review.
-
[The new drug is much more effective than ACE inhibitors in chronic heart failure].Vnitr Lek. 2015 Feb;61(2):129-33. Vnitr Lek. 2015. PMID: 25813256 Review. Czech.
Cited by
-
Acceptability and Palatability of Novel Orodispersible Minitablets of Enalapril in Children up to the Age of 6 with Heart Failure.J Clin Med. 2025 Jan 30;14(3):915. doi: 10.3390/jcm14030915. J Clin Med. 2025. PMID: 39941586 Free PMC article.
-
Population Pharmacokinetic Analysis of Enalapril and Enalaprilat in Newly Treated Children with Heart Failure: Implications for Safe Dosing of Enalapril (LENA Studies).Clin Pharmacokinet. 2025 Jul;64(7):1103-1118. doi: 10.1007/s40262-025-01520-5. Epub 2025 Jun 3. Clin Pharmacokinet. 2025. PMID: 40461938 Free PMC article. Clinical Trial.
References
-
- Bajcetic M. Complexity of Medication Regimens for Children with Heart Failure—Pilot study; Proceedings of the 56th Annual Meeting of the Association for European Paediatric and Congenital Cardiology (AEPC); Dublin, Ireland. 26–29 April 2023; Cambridge, UK: Cambridge University Press; 2003. p. 349.
-
- Castro Díez C., Khalil F., Schwender H., Dalinghaus M., Jovanovic I., Makowski N., Male C., Bajcetic M., van der Meulen M., de Wildt S.N., et al. Pharmacotherapeutic management of paediatric heart failure and ACE-I use patterns: A European survey. BMJ Paediatr. Open. 2019;3:e000365. doi: 10.1136/bmjpo-2018-000365. - DOI - PMC - PubMed
-
- Amdani S., Conway J., George K., Martinez H.R., Asante-Korang A., Goldberg C.S., Davies R.R., Miyamoto S.D., Hsu D.T., American Heart Association Council on Lifelong Congenital Heart Disease and Heart Health in the Young et al. Evaluation and Management of Chronic Heart Failure in Children and Adolescents with Congenital Heart Disease: A Scientific Statement from the American Heart Association. Circulation. 2024;150:e33–e50. doi: 10.1161/CIR.0000000000001245. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous