Association between Inflammation and New-Onset Atrial Fibrillation in Acute Coronary Syndromes
- PMID: 39274304
- PMCID: PMC11396258
- DOI: 10.3390/jcm13175088
Association between Inflammation and New-Onset Atrial Fibrillation in Acute Coronary Syndromes
Abstract
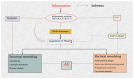
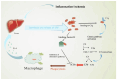
Acute coronary syndrome (ACS) is a complex clinical syndrome that encompasses acute myocardial infarction (AMI) and unstable angina (UA). Its underlying mechanism refers to coronary plaque disruption, with consequent platelet aggregation and thrombosis. Inflammation plays an important role in the progression of atherosclerosis by mediating the removal of necrotic tissue following myocardial infarction and shaping the repair processes that are essential for the recovery process after ACS. As a chronic inflammatory disorder, atherosclerosis is characterized by dysfunctional immune inflammation involving interactions between immune (macrophages, T lymphocytes, and monocytes) and vascular cells (endothelial cells and smooth muscle cells). New-onset atrial fibrillation (NOAF) is one of the most common arrhythmic complications in the setting of acute coronary syndromes, especially in the early stages, when the myocardial inflammatory reaction is at its maximum. The main changes in the atrial substrate are due to atrial ischemia and acute infarcts that can be attributed to neurohormonal factors. The high incidence of atrial fibrillation (AF) post-myocardial infarction may be secondary to inflammation. Inflammatory response and immune system cells have been involved in the initiation and development of atrial fibrillation. Several inflammatory indexes, such as C-reactive protein and interleukins, have been demonstrated to be predictive of prognosis in patients with ACS. The cell signaling activation patterns associated with fibrosis, apoptosis, and hypertrophy are forms of cardiac remodeling that occur at the atrial level, predisposing to AF. According to a recent study, the presence of fibrosis and lymphomononuclear infiltration in the atrial tissue was associated with a prior history of AF. However, inflammation may contribute to both the occurrence/maintenance of AF and its thromboembolic complications.
Keywords: acute coronary syndrome; atrial fibrillation; inflammation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Prati F., Uemura S., Souteyrand G., Virmani R., Motreff P., Di Vito L., Biondi-Zoccai G., Halperin J., Fuster V., Ozaki Y., et al. OCT-Based Diagnosis and Management of STEMI Associated with Intact Fibrous Cap. JACC Cardiovasc. Imaging. 2013;6:283–287. doi: 10.1016/j.jcmg.2012.12.007. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

