Early Clinical Experience of Finerenone in People with Chronic Kidney Disease and Type 2 Diabetes in Japan-A Multi-Cohort Study from the FOUNTAIN (FinerenOne mUltidatabase NeTwork for Evidence generAtIoN) Platform
- PMID: 39274317
- PMCID: PMC11396164
- DOI: 10.3390/jcm13175107
Early Clinical Experience of Finerenone in People with Chronic Kidney Disease and Type 2 Diabetes in Japan-A Multi-Cohort Study from the FOUNTAIN (FinerenOne mUltidatabase NeTwork for Evidence generAtIoN) Platform
Abstract
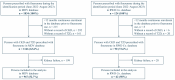
Background: In the phase 3 clinical trials FIGARO-DKD and FIDELIO-DKD, finerenone reduced the risk of cardiovascular and kidney events among people with chronic kidney disease (CKD) and type 2 diabetes (T2D). Evidence regarding finerenone use in real-world settings is limited. Methods: A retrospective cohort study (NCT06278207) using two Japanese nationwide hospital-based databases provided by Medical Data Vision (MDV) and Real World Data Co., Ltd. (RWD Co., Kyoto Japan), converted to the OMOP common data model, was conducted. Persons with CKD and T2D initiating finerenone from 1 July 2021, to 30 August 2023, were included. Baseline characteristics were described. The occurrence of hyperkalemia after finerenone initiation was assessed. Results: 1029 new users of finerenone were included (967 from MDV and 62 from RWD Co.). Mean age was 69.5 and 72.4 years with 27.3% and 27.4% being female in the MDV and RWD Co. databases, respectively. Hypertension (92 and 95%), hyperlipidemia (59 and 71%), and congestive heart failure (60 and 66%) were commonly observed comorbidities. At baseline, 80% of persons were prescribed angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers. Sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists were prescribed in 72% and 30% of the study population, respectively. The incidence proportions of hyperkalemia were 2.16 and 2.70 per 100 persons in the MDV and RWD Co. databases, respectively. There were no hospitalizations associated with hyperkalemia observed in either of the two datasets. Conclusions: For the first time, we report the largest current evidence on the clinical use of finerenone in real-world settings early after the drug authorization in Japan. This early evidence from clinical practice suggests that finerenone is used across comorbidities and comedications.
Keywords: FOUNTAIN; Observational Medical Outcomes Partnership (OMOP); chronic kidney disease; diabetes; finerenone; real world.
Conflict of interest statement
A.S. reports speaker’s bureau from AstraZeneca and Bayer. S.O. and K.Y.-R., D.R.-M., S.Y., F.L., A.F., N.G.O., D.V. are employees of Bayer. C.P.K. reports consultancy work for Abbott, Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Cara Therapeutics, CSL Vifor, GlaxoSmithKline, Prokidney, Pharmacosmos, and Takeda. K.K. reports Scholarship Grants from Japan Boehringer Ingelheim, Ono Pharmaceutical, Tanabe Mitsubishi, Kyowa, Nipro, Abbott, Terumo. This COI is for K.K.; K.K. reports Collaborative Research for Japan Boehringer Ingelheim, Boehringer Ingelheim (Germany), Taisho Pharmaceutical, Kyowa. K.K. reports Lecture Fees from Japan Boehringer Ingelheim, Japan Eli Lilly, Astellas, Tanabe Mitsubishi, MSD, Daiichi Sankyo, Sanofi, Daiichi Sankyo, Kyowa Kirin, Bayer, AstraZeneca, Otsuka, Novartis.
Figures

Similar articles
-
Evolution of Mineralocorticoid Receptor Antagonists in the Treatment of Chronic Kidney Disease Associated with Type 2 Diabetes Mellitus.Mayo Clin Proc Innov Qual Outcomes. 2022 Oct 15;6(6):536-551. doi: 10.1016/j.mayocpiqo.2022.09.002. eCollection 2022 Dec. Mayo Clin Proc Innov Qual Outcomes. 2022. PMID: 36277502 Free PMC article. Review.
-
Effectiveness of nonsteroidal mineralocorticoid receptor antagonists in patients with diabetic kidney disease.Postgrad Med. 2023 Apr;135(3):224-233. doi: 10.1080/00325481.2022.2060598. Epub 2022 Apr 20. Postgrad Med. 2023. PMID: 35392754 Review.
-
Cardiorenal benefits of finerenone: protecting kidney and heart.Ann Med. 2023 Dec;55(1):502-513. doi: 10.1080/07853890.2023.2171110. Ann Med. 2023. PMID: 36719097 Free PMC article. Review.
-
Finerenone Reduces Risk of Incident Heart Failure in Patients With Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial.Circulation. 2022 Feb 8;145(6):437-447. doi: 10.1161/CIRCULATIONAHA.121.057983. Epub 2021 Nov 13. Circulation. 2022. PMID: 34775784 Free PMC article. Clinical Trial.
-
Effects of canagliflozin versus finerenone on cardiorenal outcomes: exploratory post hoc analyses from FIDELIO-DKD compared to reported CREDENCE results.Nephrol Dial Transplant. 2022 Jun 23;37(7):1261-1269. doi: 10.1093/ndt/gfab336. Nephrol Dial Transplant. 2022. PMID: 34850173 Free PMC article. Clinical Trial.
Cited by
-
Finerenone and Estimated GFR Slope in Type 2 Diabetes and CKD.Kidney Int Rep. 2025 Apr 14;10(7):2461-2465. doi: 10.1016/j.ekir.2025.04.012. eCollection 2025 Jul. Kidney Int Rep. 2025. PMID: 40677313 Free PMC article. No abstract available.
-
Transitional changes in medication-initiator cohort profiles in persons with chronic kidney disease and type 2 diabetes-A hospital-based cohort study in Japan.Diabetes Obes Metab. 2025 Jul;27(7):3714-3724. doi: 10.1111/dom.16394. Epub 2025 Apr 21. Diabetes Obes Metab. 2025. PMID: 40259482 Free PMC article.
-
Change in Urine Albumin-Creatinine Ratio and Occurrence of Hyperkalemia in Patients Initiating Finerenone in the USA: A Cohort Study from the FOUNTAIN Platform.Nephron. 2025;149(7):371-383. doi: 10.1159/000543923. Epub 2025 Feb 27. Nephron. 2025. PMID: 40015260 Free PMC article.
-
Advances in Understanding Diabetic Kidney Disease Progression and the Mechanisms of Acupuncture Intervention.Int J Gen Med. 2024 Nov 27;17:5593-5609. doi: 10.2147/IJGM.S490049. eCollection 2024. Int J Gen Med. 2024. PMID: 39619130 Free PMC article. Review.
-
Real-world use of finerenone in patients with chronic kidney disease and type 2 diabetes based on large-scale clinical studies: FIDELIO-DKD and FIGARO-DKD.Hypertens Res. 2025 Jun;48(6):1929-1938. doi: 10.1038/s41440-025-02175-2. Epub 2025 Mar 25. Hypertens Res. 2025. PMID: 40133635 Review.
References
-
- International Diabetes Federation . IDF Diabetes Atlas. 10th ed. International Diabetes Federation; Brussels, Belgium: 2021.
-
- Wu B., Bell K., Stanford A., Kern D.M., Tunceli O., Vupputuri S., Kalsekar I., Willey V. Understanding CKD among patients with T2DM: Prevalence, temporal trends, and treatment patterns–NHAINES 2007–2012. BMJ Open Diabetes Res. Care. 2016;4:e000154. doi: 10.1136/bmjdrc-2015-000154. - DOI - PMC - PubMed
-
- Cook S., Schmedt N., Broughton J., Kalra P.A., Thomlinson L.A., Quint J.K. Characterising the burden of chronic kidney disease among people with type 2 diabetes in England: A cohort study using the Clinical Practice Research Datalink. BMJ Open. 2023;13:e065927. doi: 10.1136/bmjopen-2022-065927. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

