Early Clinical Experience of Finerenone in People with Chronic Kidney Disease and Type 2 Diabetes in Japan-A Multi-Cohort Study from the FOUNTAIN (FinerenOne mUltidatabase NeTwork for Evidence generAtIoN) Platform
- PMID: 39274317
- PMCID: PMC11396164
- DOI: 10.3390/jcm13175107
Early Clinical Experience of Finerenone in People with Chronic Kidney Disease and Type 2 Diabetes in Japan-A Multi-Cohort Study from the FOUNTAIN (FinerenOne mUltidatabase NeTwork for Evidence generAtIoN) Platform
Abstract
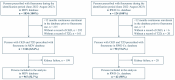
Background: In the phase 3 clinical trials FIGARO-DKD and FIDELIO-DKD, finerenone reduced the risk of cardiovascular and kidney events among people with chronic kidney disease (CKD) and type 2 diabetes (T2D). Evidence regarding finerenone use in real-world settings is limited. Methods: A retrospective cohort study (NCT06278207) using two Japanese nationwide hospital-based databases provided by Medical Data Vision (MDV) and Real World Data Co., Ltd. (RWD Co., Kyoto Japan), converted to the OMOP common data model, was conducted. Persons with CKD and T2D initiating finerenone from 1 July 2021, to 30 August 2023, were included. Baseline characteristics were described. The occurrence of hyperkalemia after finerenone initiation was assessed. Results: 1029 new users of finerenone were included (967 from MDV and 62 from RWD Co.). Mean age was 69.5 and 72.4 years with 27.3% and 27.4% being female in the MDV and RWD Co. databases, respectively. Hypertension (92 and 95%), hyperlipidemia (59 and 71%), and congestive heart failure (60 and 66%) were commonly observed comorbidities. At baseline, 80% of persons were prescribed angiotensin-converting-enzyme inhibitors or angiotensin-receptor blockers. Sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists were prescribed in 72% and 30% of the study population, respectively. The incidence proportions of hyperkalemia were 2.16 and 2.70 per 100 persons in the MDV and RWD Co. databases, respectively. There were no hospitalizations associated with hyperkalemia observed in either of the two datasets. Conclusions: For the first time, we report the largest current evidence on the clinical use of finerenone in real-world settings early after the drug authorization in Japan. This early evidence from clinical practice suggests that finerenone is used across comorbidities and comedications.
Keywords: FOUNTAIN; Observational Medical Outcomes Partnership (OMOP); chronic kidney disease; diabetes; finerenone; real world.
Conflict of interest statement
A.S. reports speaker’s bureau from AstraZeneca and Bayer. S.O. and K.Y.-R., D.R.-M., S.Y., F.L., A.F., N.G.O., D.V. are employees of Bayer. C.P.K. reports consultancy work for Abbott, Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Cara Therapeutics, CSL Vifor, GlaxoSmithKline, Prokidney, Pharmacosmos, and Takeda. K.K. reports Scholarship Grants from Japan Boehringer Ingelheim, Ono Pharmaceutical, Tanabe Mitsubishi, Kyowa, Nipro, Abbott, Terumo. This COI is for K.K.; K.K. reports Collaborative Research for Japan Boehringer Ingelheim, Boehringer Ingelheim (Germany), Taisho Pharmaceutical, Kyowa. K.K. reports Lecture Fees from Japan Boehringer Ingelheim, Japan Eli Lilly, Astellas, Tanabe Mitsubishi, MSD, Daiichi Sankyo, Sanofi, Daiichi Sankyo, Kyowa Kirin, Bayer, AstraZeneca, Otsuka, Novartis.
Figures

References
-
- International Diabetes Federation . IDF Diabetes Atlas. 10th ed. International Diabetes Federation; Brussels, Belgium: 2021.
-
- Wu B., Bell K., Stanford A., Kern D.M., Tunceli O., Vupputuri S., Kalsekar I., Willey V. Understanding CKD among patients with T2DM: Prevalence, temporal trends, and treatment patterns–NHAINES 2007–2012. BMJ Open Diabetes Res. Care. 2016;4:e000154. doi: 10.1136/bmjdrc-2015-000154. - DOI - PMC - PubMed
-
- Cook S., Schmedt N., Broughton J., Kalra P.A., Thomlinson L.A., Quint J.K. Characterising the burden of chronic kidney disease among people with type 2 diabetes in England: A cohort study using the Clinical Practice Research Datalink. BMJ Open. 2023;13:e065927. doi: 10.1136/bmjopen-2022-065927. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

