Use of Eltrombopag to Improve Thrombocytopenia and Tranfusion Requirement in Anti-CD19 CAR-T Cell-Treated Patients
- PMID: 39274330
- PMCID: PMC11396136
- DOI: 10.3390/jcm13175117
Use of Eltrombopag to Improve Thrombocytopenia and Tranfusion Requirement in Anti-CD19 CAR-T Cell-Treated Patients
Abstract
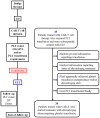
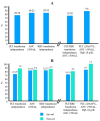
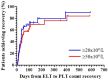
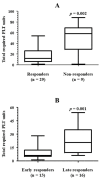
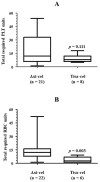
Background/Objectives: Immune effector cell-associated hematotoxicity (ICAHT) is a frequent adverse event after chimeric antigen receptor (CAR)-T cell therapy. Grade ≥ 3 thrombocytopenia occurs in around one-third of patients, and many of them become platelet transfusion-dependent. Eltrombopag is a thrombopoietin receptor agonist (TPO-RA) able to accelerate megakaryopoiesis, which has been used successfully in patients with bone marrow failure and immune thrombocytopenia (ITP). Its role in managing thrombocytopenia and other cytopenias in CAR-T cell-treated patients has been scarcely addressed. Our aim was to report the safety and efficacy of this approach in patients included in the Spanish Group for Hematopoietic Transplantation and Cellular Therapy (GETH-TC) registry. Methods: This is a retrospective, multicenter, observational study. Patients who developed platelet transfusion dependence subsequently to CAR-T cells and received eltrombopag to improve platelet counts were recruited in 10 Spanish hospitals. Results: Thirty-eight patients were enrolled and followed up for a median (interquartile range [IQR]) of 175 (99, 489) days since CAR-T cell infusion. At the moment eltrombopag was indicated, 18 patients had thrombocytopenia and another severe cytopenia, while 8 patients had severe pancytopenia. After 32 (14, 38) days on eltrombopag, 29 (76.3%) patients recovered platelet transfusion independence. The number of platelet units transfused correlated with the time needed to restore platelet counts higher than 20 × 109/L (Rho = 0.639, p < 0.001). Non-responders to eltrombopag required more platelet units (58 [29, 69] vs. 12 [6, 26] in responders, p = 0.002). Nineteen out of twenty-three (82.6%) patients recovered from severe neutropenia after 22 (11, 31) days on eltrombopag. Twenty-nine out of thirty-five (82.9%) patients recovered red blood cell (RBC) transfusion independence after 29 (17, 44) days. Seven patients recovered all cell lineages while on treatment. No thromboembolic events were reported. Only two transient toxicities (cholestasis, hyperbilirubinemia) were reported during eltrombopag treatment, none of which compelled permanent drug withdrawal. Conclusions: Eltrombopag could be safely used to manage thrombocytopenia and accelerate transfusion independence in CAR-T cell-treated patients.
Keywords: CAR-T; adverse events; eltrombopag; neutropenia; pan-cytopenia; platelet transfusion; red blood cell transfusion; thrombocytopenia; thrombopoietin receptor agonist.
Conflict of interest statement
MEMC reports no conflict for this paper.
Figures


References
LinkOut - more resources
Full Text Sources

