IL-17 and IL-23 Inhibitors Have the Fastest Time to Meaningful Clinical Response for Plaque Psoriasis: A Network Meta-Analysis
- PMID: 39274352
- PMCID: PMC11396496
- DOI: 10.3390/jcm13175139
IL-17 and IL-23 Inhibitors Have the Fastest Time to Meaningful Clinical Response for Plaque Psoriasis: A Network Meta-Analysis
Abstract
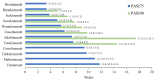
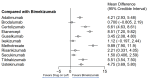
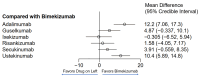
Background/Objectives: Several treatment options with differing mechanisms exist for plaque psoriasis. The objective of this analysis was to compare the time to onset of action among the available systemic therapies for plaque psoriasis. Methods: Randomized controlled trials that investigated two or more therapeutics for the management of moderate to severe plaque psoriasis were included. A weighted average time for 50% of patients to reach PASI75 and PAI90 with each of the therapeutics was calculated. A network meta-analysis was performed to determine which therapeutics were significantly faster in time to meaningful clinical response than others. Results: IL-17 inhibitors had the shortest time to achieve PASI75 and PASI90 followed by risankizumab in the weighted mean analysis. In the meta-analysis, the fastest time to PASI75 was seen with bimekizumab, brodalumab and ixekizumab. No significant (p < 0.05) difference was seen in the time to meaningful clinical response between these drugs; however, bimekizumab was significantly faster in time to PASI75 among the remaining therapeutics. In the meta-analysis for PASI90, the fastest time was seen with ixekizumab, bimekizumab, risankizumab, secukinumab and guselkumab with no significant differences in between these therapeutics. However, bimekizumab was significantly faster than the remaining therapeutics for PASI90. Conclusions: IL-17 and IL-23 inhibitors may be considered as requiring the shortest time for meaningful clinical response in plaque psoriasis. In addition to the time to onset, the safety profile of each drug needs to be considered when deciding on a therapeutic to initiate.
Keywords: PASI-75; PASI-90; network meta-analysis.
Conflict of interest statement
Alan Fleischer is a consultant for Bluefin and Incyte (fees). He is an investigator for Amgen, Bayer, Biogen, Cara, Galderma, Incyte, Trevi and UCB (research support). Other authors declare no conflicts of interest.
Figures
References
-
- Badri T., Kumar P., Oakley A.M. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. [(accessed on 17 July 2024)]. Plaque Psoriasis. Available online: http://www.ncbi.nlm.nih.gov/books/NBK430879/ - PubMed
-
- Egeberg A., Andersen Y.M.F., Halling-Overgaard A., Alignahi F., Thyssen J.P., Burge R., Mallbris L. Systematic review on rapidity of onset of action for interleukin-17 and interleukin-23 inhibitors for psoriasis. J. Eur. Acad. Dermatol. Venereol. 2020;34:39–46. doi: 10.1111/jdv.15920. - DOI - PubMed
-
- Yao C.J., Lebwohl M.G. Onset of Action of Antipsoriatic Drugs for Moderate-to-Severe Plaque Psoriasis: An Update. J. Drugs Dermatol. 2019;18:229–233. - PubMed
LinkOut - more resources
Full Text Sources

