Clinical Trial: A Pragmatic Randomised Controlled Study to Assess the Effectiveness of Two Patient Management Strategies in Mild to Moderate Ulcerative Colitis-The OPTIMISE Study
- PMID: 39274360
- PMCID: PMC11395821
- DOI: 10.3390/jcm13175147
Clinical Trial: A Pragmatic Randomised Controlled Study to Assess the Effectiveness of Two Patient Management Strategies in Mild to Moderate Ulcerative Colitis-The OPTIMISE Study
Abstract
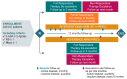
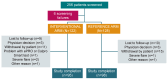
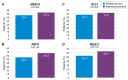
Background: Current management of mild-to-moderate ulcerative colitis (UC) involves monitoring clinical markers of disease activity, such as stool frequency (SF) and rectal bleeding (RB), and adjusting treatment accordingly. Our aim was to assess whether targeting treatment based on faecal calprotectin (FC) levels (treat-to-target; T2T) provides greater UC disease control versus a symptom-based approach. Methods: This was a pragmatic, randomised (1:1) controlled study of patients with mild-to-moderate UC (global Mayo score 2-6) treated with ≤2.4 g/day 5-aminosalicylic acid that compared the effectiveness of two management strategies with (interventional arm) and without (reference arm) FC home monitoring over 12 months of follow-up. Treatment was optimised in the interventional arm using FC values and clinical symptoms (PRO-2), while the reference arm used only PRO-2. Results: 193 patients completed the study. No significant difference was found for the primary endpoint (Mayo Endoscopic Subscore [MES] = 0 at 12 months). A numerical advantage for the interventional arm over the reference arm for the primary endpoint (37.0% vs. 33.4%, respectively) and for MES ≤ 1, RB = 0, and SF ≤ 1 at 12 months was found following imputation for missing data. The composite endpoint of MES = 0, RB = 0, and SF ≤ 1 at 12 months was achieved at a significantly higher rate in the interventional arm than the reference arm (effect size [ES]: 0.17, 95% CI 0.02-0.32; p < 0.05). A similar result was obtained for MES ≤ 1, RB = 0 and SF ≤ 1 (ES: 0.22; 95% CI 0.07-0.37; p < 0.05). Conclusions: T2T using FC monitoring was effective in patients with mild-to-moderate UC at 12 months. Further longer-term studies are required to confirm the results.
Keywords: faecal calprotectin; inflammatory bowel disease; tight monitoring; treat-to-target; ulcerative colitis.
Conflict of interest statement
GF served as a speaker and advisory board member for Takeda, Abbvie, Janssen, Pfizer, Celltrion, Sandoz, Ferring, Galapagos, BMS, and STADA. FD has served as a speaker for Sandoz, Janssen, Galapagos, Takeda, and Omega Pharma; he has also served as an advisory board member for Abbvie, Ferring, Galapagos, Janssen, and Nestlè. SD has served as a speaker, consultant, and advisory board member for Schering-Plough, AbbVie, Actelion, Alphawasserman, AstraZeneca, Cellerix, Cosmo Pharmaceuticals, Ferring, Genentech, Grunenthal, Johnson and Johnson, Millenium Takeda, MSD, Nikkiso Europe GmbH, Novo Nordisk, Nycomed, Pfizer, Pharmacosmos, UCB Pharma, and Vifor. LG served as a speaker for Abbvie (related to gastro/jejunostomy for Duodopa, not for IBD-associated medication). BK received a grant from Ferring for the study and research conducted. RLW has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Ferring, Pfizer, Galapagos, AbbVie, and Janssen. KP, AU, and BC are employees of Ferring Pharmaceuticals. TV served Takeda, invited lectures, advisory board. EV consulting fees at Abbott and Coloplast. LPB has served as a speaker, consultant, and advisory board member for Merck, Abbvie, Janssen, Genentech, Mitsubishi, Ferring, Norgine, Tillots, Vifor, Hospira/Pfizer, Celltrion, Takeda, Biogaran, Boerhinger-Ingelheim, Lilly, HAC Pharma, Index Pharmaceuticals, Amgen, Sandoz, Forward Pharma GmbH, Celgene, Biogen, Lycera, Samsung Bioepis, and Theravance. Kristine Paridaens was employed by the company Ferring International Center S.A, Asiya Ugur, Brian Clark are employed by the company Ferring Pharmaceuticals. DS, MWJ, PD, GF, MF, MW, MK, and MW have nothing to disclose.
Figures


References
-
- Magro F., Gionchetti P., Eliakim R., Ardizzone S., Armuzzi A., Barreiro-de Acosta M., Burisch J., Gecse K.B., Hart A.L., Hindryckx P., et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohns Colitis. 2017;11:649–670. doi: 10.1093/ecco-jcc/jjx008. - DOI - PubMed
-
- Harbord M., Eliakim R., Bettenworth D., Karmiris K., Katsanos K., Kopylov U., Kucharzik T., Molnár T., Raine T., Sebastian S., et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 2: Current Management. J. Crohns Colitis. 2017;11:769–784. doi: 10.1093/ecco-jcc/jjx009. - DOI - PubMed
-
- Lewis J.D., Parlett L.E., Jonsson Funk M.L., Brensinger C., Pate V., Wu Q., Dawwas G.K., Weiss A., Constant B.D., McCauley M., et al. Incidence, Prevalence, and Racial and Ethnic Distribution of Inflammatory Bowel Disease in the United States. Gastroenterology. 2023;165:1197–1205.e2. doi: 10.1053/j.gastro.2023.07.003. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

