Efficient Hydroxyapatite Extraction from Salmon Bone Waste: An Improved Lab-Scaled Physico-Chemico-Biological Process
- PMID: 39274852
- PMCID: PMC11396111
- DOI: 10.3390/molecules29174002
Efficient Hydroxyapatite Extraction from Salmon Bone Waste: An Improved Lab-Scaled Physico-Chemico-Biological Process
Abstract
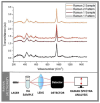
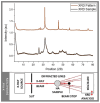
The demand for novel tissue grafting and regenerative wound care biomaterials is growing as traditional options often fall short in biocompatibility, functional integration with human tissue, associated cost(s), and sustainability. Salmon aquaculture generates significant volumes of waste, offering a sustainable opportunity for biomaterial production, particularly in osteo-conduction/-induction, and de novo clinical/surgical bone regeneration. Henceforth, this study explores re-purposing salmon waste through a standardized pre-treatment process that minimizes the biological waste content, followed by a treatment stage to remove proteins, lipids, and other compounds, resulting in a mineral-rich substrate. Herein, we examined various methods-alkaline hydrolysis, calcination, and NaOH hydrolysis-to better identify and determine the most efficient and effective process for producing bio-functional nano-sized hydroxyapatite. Through comprehensive chemical, physical, and biological assessments, including Raman spectroscopy and X-ray diffraction, we also optimized the extraction process. Our modified and innovative alkaline hydrolysis-calcination method yielded salmon-derived hydroxyapatite with a highly crystalline structure, an optimal Ca/P ratio, and excellent biocompatibility. The attractive nano-scale cellular/tissular properties and favorable molecular characteristics, particularly well-suited for bone repair, are comparable to or even surpass those of synthetic, human, bovine, and porcine hydroxyapatite, positioning it as a promising candidate for use in tissue engineering, wound healing, and regenerative medicine indications.
Keywords: biomaterial; bone repair; hydroxyapatite; osseoregenerate; process; salmon bone; waste.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Haidar Z.S. nanoBONE: Re-visiting Osseo-Recons-truction and -Repair... with a nanoTwist. J. Oral Res. 2021;10:1–6. doi: 10.17126/joralres.2021.047. - DOI
-
- Zumarán C.C., Parra M.V., Olate S.A., Fernández E.G., Muñoz F.T., Haidar Z.S. The 3 R’s for Platelet-Rich Fibrin: A “Super” Tri-Dimensional Biomaterial for Contemporary Naturally-Guided Oro-Maxillo-Facial Soft and Hard Tissue Repair, Reconstruction and Regeneration. Materials. 2018;11:1293. doi: 10.3390/ma11081293. - DOI - PMC - PubMed
-
- Damsaz M., Castagnoli C.Z., Eshghpour M., Alamdari D.H., Alamdari A.H., Noujeim Z.E.F., Haidar Z.S. Evidence-Based Clinical Efficacy of Leukocyte and Platelet-Rich Fibrin in Maxillary Sinus Floor Lift, Graft and Surgical Augmentation Procedures. Front. Surg. 2020;7:537138. doi: 10.3389/fsurg.2020.537138. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

