A Convenient Synthesis of Short α-/β-Mixed Peptides as Potential α-Amylase Inhibitors
- PMID: 39274877
- PMCID: PMC11396456
- DOI: 10.3390/molecules29174028
A Convenient Synthesis of Short α-/β-Mixed Peptides as Potential α-Amylase Inhibitors
Abstract
Over the last decades, the increased incidence of metabolic disorders, such as type two diabetes and obesity, has motivated researchers to investigate new enzyme inhibitors. Inhibition of the α-amylase enzyme is one therapeutic approach in lowering glucose levels in the blood to manage diabetes mellitus. The objective of this study was to synthesize short α-/β-mixed peptides in the solution phase. The Boc-protected α-L-leucine was converted to β-analogue by using Arndt-Eistert synthesis with the advantage of no racemization and retention of configuration. Three novel short peptides were successfully synthesized: N(Boc)-Gly-β-Leu-OCH3(14), N(Boc)-O(Bz)α-Ser-β-Leu-OCH3(16), and N(Boc)-O(Bz)-α-Tyr-α-Gly-β-Leu-OCH3(17), characterized by FTIR and 1H NMR analysis. The synthesized peptide 16 showed highest inhibitory activity (45.22%) followed by peptide 14 (18.51%) and peptide 17 (17.05%), respectively. Intriguingly, peptide 16 showed higher inhibition on α-amylase compared with other α-/β-mixed peptides.
Keywords: Arndt–Eistert synthesis; postprandial hyperglycemia; α-/β-mixed peptides; β-amino acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



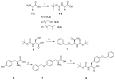

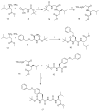
References
-
- Makkar F., Chakraborty K. Antidiabetic and anti-inflammatory potential of sulphated polygalactans from red seaweeds Kappaphycus alvarezii and Gracilaria opuntia. Int. J. Food Prop. 2017;20:1326–1337. doi: 10.1080/10942912.2016.1209216. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

