Bioactive Molecules Delivery through Ferritin Nanoparticles: Sum Up of Current Loading Methods
- PMID: 39274893
- PMCID: PMC11396501
- DOI: 10.3390/molecules29174045
Bioactive Molecules Delivery through Ferritin Nanoparticles: Sum Up of Current Loading Methods
Abstract
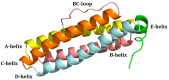
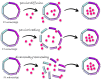
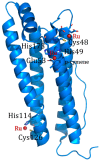
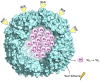
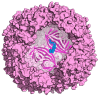
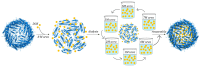
Ferritin (Ft) is a protein with a peculiar three-dimensional architecture. It is characterized by a hollow cage structure and is responsible for iron storage and detoxification in almost all living organisms. It has attracted the interest of the scientific community thanks to its appealing features, such as its nano size, thermal and pH stability, ease of functionalization, and low cost for large-scale production. Together with high storage capacity, these properties qualify Ft as a promising nanocarrier for the development of delivery systems for numerous types of biologically active molecules. In this paper, we introduce the basic structural and functional aspects of the protein, and summarize the methods employed to load bioactive molecules within the ferritin nanocage.
Keywords: bioactive molecules; drug delivery; encapsulation; ferritin nanocages; loading protocols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

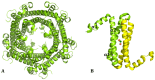



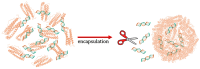


Similar articles
-
Human ferritin nanocarriers for drug-delivery: A molecular view of the disassembly process.Int J Biol Macromol. 2024 Oct;277(Pt 2):134373. doi: 10.1016/j.ijbiomac.2024.134373. Epub 2024 Jul 31. Int J Biol Macromol. 2024. PMID: 39094874
-
Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery.Acc Chem Res. 2021 Sep 7;54(17):3313-3325. doi: 10.1021/acs.accounts.1c00267. Epub 2021 Aug 20. Acc Chem Res. 2021. PMID: 34415728 Review.
-
AB loop engineered ferritin nanocages for drug loading under benign experimental conditions.Chem Commun (Camb). 2019 Oct 10;55(82):12344-12347. doi: 10.1039/c9cc05247j. Chem Commun (Camb). 2019. PMID: 31556881
-
Ferritin cage for encapsulation and delivery of bioactive nutrients: From structure, property to applications.Crit Rev Food Sci Nutr. 2017 Nov 22;57(17):3673-3683. doi: 10.1080/10408398.2016.1149690. Crit Rev Food Sci Nutr. 2017. PMID: 26980693 Review.
-
Heat sensitive E-helix cut ferritin nanocages for facile and high-efficiency loading of doxorubicin.Int J Biol Macromol. 2023 Dec 31;253(Pt 3):126973. doi: 10.1016/j.ijbiomac.2023.126973. Epub 2023 Sep 18. Int J Biol Macromol. 2023. PMID: 37729988
Cited by
-
Controlling nanocage assembly, towards developing a one-health "plug & play" platform for targeted therapy.Chem Commun (Camb). 2025 Aug 28;61(71):13221-13235. doi: 10.1039/d5cc03592a. Chem Commun (Camb). 2025. PMID: 40824119 Free PMC article. Review.
References
-
- He D., Hughes S., Vanden-Hehir S., Georgiev A., Altenbach K., Tarrant E., Mackay C.L., Waldron K.J., Clarke D.J., Marles-Wright J. Structural Characterization of Encapsulated Ferritin Provides Insight into Iron Storage in Bacterial Nanocompartments. eLife. 2016;5:e18972. doi: 10.7554/eLife.18972. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

