Stability Evaluation and Pharmacokinetic Profiling of Vepdegestrant in Rodents Using Liquid Chromatography-Tandem Mass Spectrometry
- PMID: 39274896
- PMCID: PMC11396746
- DOI: 10.3390/molecules29174048
Stability Evaluation and Pharmacokinetic Profiling of Vepdegestrant in Rodents Using Liquid Chromatography-Tandem Mass Spectrometry
Abstract
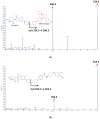
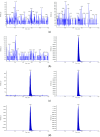
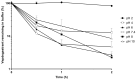
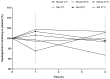
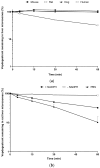
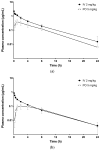
Vepdegestrant (formerly ARV-471), a novel proteolysis-targeting chimera (PROTAC), targets estrogen receptor alpha (ERα) for degradation, offering a promising option to treat advanced ER-positive breast cancer. We developed and validated a sensitive and rapid liquid chromatography-tandem mass spectrometry method to quantify vepdegestrant in rodent plasma using bavdegalutamide (formerly ARV-110) as an internal standard. Plasma samples were prepared with protein precipitation using acetonitrile and analyzed using reverse-phase C18 columns and a mobile phase of 10 mM ammonium formate in distilled water and acetonitrile. The method demonstrated linearity from 1 to 1000 ng/mL in mouse and rat plasma, meeting all validation criteria, and successfully applied to in vivo and in vitro studies. Pharmacokinetic analysis revealed low-to-moderate clearance (313.3, 1053 mL/h/kg) and oral bioavailability (17.91, 24.12%) of vepdegestrant in mice and rats, respectively. It was unstable in buffer solutions across pH 2-10 and in phosphate-buffered saline (pH 7.4), likely due to adsorption, but remained stable in mouse and rat plasma at varying temperatures. In liver microsomes, vepdegestrant exhibited moderate stability in rats but was stable in mice, dogs, and humans. These findings enhance the understanding of pharmacokinetic properties of vepdegestrant supporting further development of PROTAC drugs.
Keywords: ARV-471; LC-MS/MS; PROTAC; pharmacokinetics; stability; validation; vepdegestrant.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- International Agency for Research on Cancer. [(accessed on 25 June 2024)]. Available online: https://gco.iarc.who.int/media/globocan/factsheets/cancers/20-breast-fac....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

