Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens
- PMID: 39274911
- PMCID: PMC11396672
- DOI: 10.3390/molecules29174065
Agents Targeting the Bacterial Cell Wall as Tools to Combat Gram-Positive Pathogens
Abstract
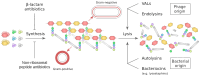
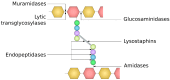
The cell wall is an indispensable element of bacterial cells and a long-known target of many antibiotics. Penicillin, the first discovered beta-lactam antibiotic inhibiting the synthesis of cell walls, was successfully used to cure many bacterial infections. Unfortunately, pathogens eventually developed resistance to it. This started an arms race, and while novel beta-lactams, either natural or (semi)synthetic, were discovered, soon upon their application, bacteria were developing resistance. Currently, we are facing the threat of losing the race since more and more multidrug-resistant (MDR) pathogens are emerging. Therefore, there is an urgent need for developing novel approaches to combat MDR bacteria. The cell wall is a reasonable candidate for a target as it differentiates not only bacterial and human cells but also has a specific composition unique to various groups of bacteria. This ensures the safety and specificity of novel antibacterial agents that target this structure. Due to the shortage of low-molecular-weight candidates for novel antibiotics, attention was focused on peptides and proteins that possess antibacterial activity. Here, we describe proteinaceous agents of various origins that target bacterial cell wall, including bacteriocins and phage and bacterial lysins, as alternatives to classic antibiotic candidates for antimicrobial drugs. Moreover, advancements in protein chemistry and engineering currently allow for the production of stable, specific, and effective drugs. Finally, we introduce the concept of selective targeting of dangerous pathogens, exemplified by staphylococci, by agents specifically disrupting their cell walls.
Keywords: Gram-positive bacteria; Staphylococcus aureus; antibiotic; bacterial cell wall; endolysin; lysostaphin; peptidoglycan; peptidoglycan hydrolase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Constructing and deconstructing the bacterial cell wall.Protein Sci. 2020 Mar;29(3):629-646. doi: 10.1002/pro.3737. Epub 2019 Nov 20. Protein Sci. 2020. PMID: 31747090 Free PMC article. Review.
-
Antimicrobial Peptides Derived from Bacteria: Classification, Sources, and Mechanism of Action against Multidrug-Resistant Bacteria.Int J Mol Sci. 2024 Oct 8;25(19):10788. doi: 10.3390/ijms251910788. Int J Mol Sci. 2024. PMID: 39409116 Free PMC article. Review.
-
Fighting infections due to multidrug-resistant Gram-positive pathogens.Clin Microbiol Infect. 2009 Mar;15(3):209-11. doi: 10.1111/j.1469-0691.2009.02737.x. Clin Microbiol Infect. 2009. PMID: 19335367
-
Novel antibacterial agents for the treatment of serious Gram-positive infections.Expert Opin Investig Drugs. 2003 Mar;12(3):379-99. doi: 10.1517/13543784.12.3.379. Expert Opin Investig Drugs. 2003. PMID: 12605562 Review.
-
Facilitation of horizontal transfer of antimicrobial resistance by transformation of antibiotic-induced cell-wall-deficient bacteria.Med Hypotheses. 2003 Oct;61(4):503-8. doi: 10.1016/s0306-9877(03)00205-6. Med Hypotheses. 2003. PMID: 13679020
Cited by
-
Extracellular vesicles enhance the efficacy of ceftiofur against intracellular bacterial infections.Synth Syst Biotechnol. 2025 Jul 3;10(4):1200-1207. doi: 10.1016/j.synbio.2025.07.001. eCollection 2025 Dec. Synth Syst Biotechnol. 2025. PMID: 40727487 Free PMC article.
-
Assessing the Antibiotic Resistance in Food Lactic Acid Bacteria: Risks in the Era of Widespread Probiotic Use.Food Sci Nutr. 2025 Jul 31;13(8):e70740. doi: 10.1002/fsn3.70740. eCollection 2025 Aug. Food Sci Nutr. 2025. PMID: 40755505 Free PMC article. Review.
-
The impact of metagenomic analysis on the discovery of novel endolysins.Appl Microbiol Biotechnol. 2025 May 24;109(1):126. doi: 10.1007/s00253-025-13513-2. Appl Microbiol Biotechnol. 2025. PMID: 40411603 Free PMC article. Review.
-
Antibacterial activity of promising nanostructured cesium oxide.Discov Nano. 2025 Aug 18;20(1):140. doi: 10.1186/s11671-025-04327-2. Discov Nano. 2025. PMID: 40824504 Free PMC article.
-
Novel Antibacterial Approaches and Therapeutic Strategies.Antibiotics (Basel). 2025 Apr 15;14(4):404. doi: 10.3390/antibiotics14040404. Antibiotics (Basel). 2025. PMID: 40298586 Free PMC article. Review.
References
-
- WHO . WHO Bacterial Priority Pathogens List, 2024. WHO; Geneva, Switzerland: 2024.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

