Recent Advances in Fluorescent Polyimides
- PMID: 39274921
- PMCID: PMC11397098
- DOI: 10.3390/molecules29174072
Recent Advances in Fluorescent Polyimides
Abstract
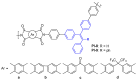
Polyimide (PI) refers to a type of high-performance polymer containing imide rings in the main chain, which has been widely used in fields of aerospace, microelectronic and photonic devices, gas separation technology, and so on. However, traditional aromatic PIs are, in general, the inefficient fluorescence or even no fluorescence, due to the strong inter- and intramolecular charge transfer (CT) interactions causing unavoidable fluorescence quenching, which greatly restricts their applications as light-emitting functional layers in the fabrication of organic light-emitting diode (OLED) devices. As such, the development of fluorescent PIs with high fluorescence quantum efficiency for their application fields in the OLED is an important research direction in the near future. In this review, we provide a comprehensive overview of fluorescent PIs as well as the methods to improve the fluorescence quantum efficiency of PIs. It is anticipated that this review will serve as a valuable reference and offer guidance for the design and development of fluorescent PIs with high fluorescence quantum efficiency, ultimately fostering further progress in OLED research.
Keywords: CT interactions; fluorescence; fluorescent polyimide; structure.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures










































References
-
- Wu A., Akagi T., Jikei M., Kakimoto M.-A., Imai Y., Ukishima S., Takahashi Y. New fluorescent polyimides for electrolumi-nescent devices based on 2,5-distyrylpyrazine. Thin Solid Film. 1996;273:214–217. doi: 10.1016/0040-6090(95)06780-9. - DOI
-
- Sun N., Zou Q., Chen W., Zheng Y., Sun K., Li C., Han Y., Bai L., Wei C., Lin J., et al. Fluorene pen-dant-functionalization of poly(N-vinylcarbazole) as deep-blue fluorescent and host materials for polymer light-emitting diodes. Chin. Chem. Lett. 2023;34:108078. doi: 10.1016/j.cclet.2022.108078. - DOI
-
- Giuffreda E., Side D.D., Krasa J., Nassisi V. Polarization of plastic targets by laser ablation. J. Instrum. 2016;11:C05004. doi: 10.1088/1748-0221/11/05/C05004. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

