Gypensapogenin A-Liposomes Efficiently Ameliorates Hepatocellular Lipid Accumulation via Activation of FXR Receptor
- PMID: 39274927
- PMCID: PMC11397205
- DOI: 10.3390/molecules29174080
Gypensapogenin A-Liposomes Efficiently Ameliorates Hepatocellular Lipid Accumulation via Activation of FXR Receptor
Abstract
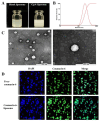
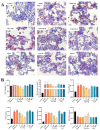
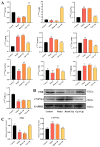
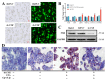
Non-alcoholic fatty liver disease (NAFLD) is one of the most common metabolic diseases encountered in clinical practice, which is characterized by the excessive accumulation of triglycerides (steatosis), and a variety of metabolic abnormalities including lipid metabolism and bile acid metabolism are closely related to NAFLD. In China, Gynostemma pentaphyllum is used as functional food and Chinese medicine to treat various diseases, especially NAFLD, for a long time. However, the active components that exert the main therapeutic effects and their mechanisms remain unclear. In this study, Gypensapogenin A was isolated from the total saponins of G. pentaphyllum and prepared as a liposomal delivery system. Gypensapogenin A liposomes could activate FXR, inhibit the expression of CYP7A1 and CYP8B1, increase the expression of CYP27A1, modulate the ratio of CA and CDCA, decrease the content of CA, and increase the content of CDCA, thus forming a virtuous cycle of activating FXR to play a role in lowering blood lipid levels.
Keywords: FXR; Gynostemma pentaphyllum; Gypensapogenin A; bile acids; hyperlipidemia.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures




Similar articles
-
Ganoderma Lucidum Polysaccharide Peptide Alleviates Hepatoteatosis via Modulating Bile Acid Metabolism Dependent on FXR-SHP/FGF.Cell Physiol Biochem. 2018;49(3):1163-1179. doi: 10.1159/000493297. Epub 2018 Sep 7. Cell Physiol Biochem. 2018. PMID: 30196282
-
Schaftoside alleviates HFD-induced hepatic lipid accumulation in mice via upregulating farnesoid X receptor.J Ethnopharmacol. 2020 Jun 12;255:112776. doi: 10.1016/j.jep.2020.112776. Epub 2020 Mar 20. J Ethnopharmacol. 2020. PMID: 32205261
-
Medium chain triglycerides alleviate non-alcoholic fatty liver disease through bile acid-mediated FXR signaling pathway: A comparative study with common vegetable edible oils.J Food Sci. 2024 Dec;89(12):10171-10180. doi: 10.1111/1750-3841.17565. Epub 2024 Dec 12. J Food Sci. 2024. PMID: 39668111
-
Bile acids are nutrient signaling hormones.Steroids. 2014 Aug;86:62-8. doi: 10.1016/j.steroids.2014.04.016. Epub 2014 May 10. Steroids. 2014. PMID: 24819989 Free PMC article. Review.
-
Bile acids mediated potential functional interaction between FXR and FATP5 in the regulation of Lipid Metabolism.Int J Biol Sci. 2020 Jun 14;16(13):2308-2322. doi: 10.7150/ijbs.44774. eCollection 2020. Int J Biol Sci. 2020. PMID: 32760200 Free PMC article. Review.
Cited by
-
The Connection Between Lipid Metabolism in the Heart and Liver of Wuzhishan Pigs.Biomolecules. 2025 Jul 16;15(7):1024. doi: 10.3390/biom15071024. Biomolecules. 2025. PMID: 40723896 Free PMC article.
-
The Prebiotic Effect of Kaempferol in Regulating Bile Acid Metabolism.Food Sci Nutr. 2025 Feb 24;13(3):e70023. doi: 10.1002/fsn3.70023. eCollection 2025 Mar. Food Sci Nutr. 2025. PMID: 40008236 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

