Electrospun PVP Fibers as Carriers of Ca2+ Ions to Improve the Osteoinductivity of Titanium-Based Dental Implants
- PMID: 39275029
- PMCID: PMC11397674
- DOI: 10.3390/molecules29174181
Electrospun PVP Fibers as Carriers of Ca2+ Ions to Improve the Osteoinductivity of Titanium-Based Dental Implants
Abstract
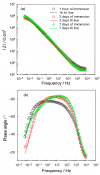
Although titanium and its alloys are widely used as dental implants, they cannot induce the formation of new bone around the implant, which is a basis for the functional integrity and long-term stability of implants. This study focused on the functionalization of the titanium/titanium oxide surface as the gold standard for dental implants, with electrospun composite fibers consisting of polyvinylpyrrolidone and Ca2+ ions. Polymer fibers as carriers of Ca2+ ions should gradually dissolve, releasing Ca2+ ions into the environment of the implant when it is immersed in a model electrolyte of artificial saliva. Scanning electron microscopy, energy dispersive X-ray spectroscopy and attenuated total reflectance Fourier transform infrared spectroscopy confirmed the successful formation of a porous network of composite fibers on the titanium/titanium oxide surface. The mechanism of the formation of the composite fibers was investigated in detail by quantum chemical calculations at the density functional theory level based on the simulation of possible molecular interactions between Ca2+ ions, polymer fibers and titanium substrate. During the 7-day immersion of the functionalized titanium in artificial saliva, the processes on the titanium/titanium oxide/composite fibers/artificial saliva interface were monitored by electrochemical impedance spectroscopy. It can be concluded from all the results that the composite fibers formed on titanium have application potential for the development of osteoinductive and thus more biocompatible dental implants.
Keywords: Ca2+ ions; DFT; EIS; electrospinning; polyvinylpyrrolidone; titanium.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

