The Skin Histopathology of Pro- and Parabiotics in a Mouse Model of Atopic Dermatitis
- PMID: 39275219
- PMCID: PMC11397434
- DOI: 10.3390/nu16172903
The Skin Histopathology of Pro- and Parabiotics in a Mouse Model of Atopic Dermatitis
Abstract
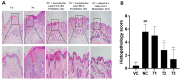
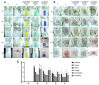
As it has been revealed that the activation of human immune cells through the activity of intestinal microorganisms such as pro- and prebiotics plays a vital role, controlling the proliferation of beneficial bacteria and suppressing harmful bacteria in the intestine has become essential. The importance of probiotics, especially for skin health and the immune system, has led to the emergence of products in various forms, including probiotics, prebiotics, and parabiotics. In particular, atopic dermatitis (AD) produces hypersensitive immunosuppressive substances by promoting the differentiation and activity of immune regulatory T cells. As a result, it has been in the Th1 and Th2 immune balance through a mechanism that suppresses skin inflammation or allergic immune responses caused by bacteria. Furthermore, an immune mechanism has recently emerged that simultaneously controls the expression of IL-17 produced by Th17. Therefore, the anti-atopic effect was investigated by administering doses of anti-atopic candidate substances (Lactobacilus sakei CVL-001, Lactobacilus casei MCL, and Lactobacilus sakei CVL-001 Lactobacilus casei MCL mixed at a ratio of 4:3) in an atopy model using 2,4-dinitrochlorobenzene and observing symptom changes for 2 weeks to confirm the effect of pro-, para-, and mixed biotics on AD. First, the body weight and feed intake of the experimental animals were investigated, and total IgG and IgM were confirmed through blood biochemical tests. Afterward, histopathological staining was performed using H&E staining, Toluidine blue staining, Filaggrin staining, and CD8 antibody staining. In the treatment group, the hyperproliferation of the epidermal layer, the inflammatory cell infiltration of the dermal layer, the expression of CD8, the expression of filaggrin, and the secretion of mast cells were confirmed to be significantly reduced. Lastly, small intestine villi were observed through a scanning microscope, and scoring evaluation was performed through skin damage. Through these results, it was confirmed that AD was reduced when treated with pro-, para-, and mixed biotics containing probiotics and parabiotics.
Keywords: Lactobacilus casei MCL; Lactobacilus sakei CVL-001; anti-inflammation; atopic dermatitis (AD); parabiotics; probiotics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Perkin M.R., Strachan D.P., Williams H.C., Kennedy C.T.C., Golding J., The ALSPAC Study Team Natural history of atopic dermatitis and its relationship to serum total immunoglobulin E in a population-based birth cohort study. Pediatr. Allergy Immunol. 2004;15:221–229. doi: 10.1111/j.1399-3038.2004.00160.x. - DOI - PubMed
-
- Chiesa Fuxench Z.C. Management of Atopic Dermatitis: Methods and Challenges. Springer; Berlin/Heidelberg, Germany: 2017. Atopic dermatitis: Disease background and risk factors; pp. 11–19.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

