Adjusted versus Targeted Fortification in Extremely Low Birth Weight Preterm Infants: Fortin Study-A Randomized Clinical Trial
- PMID: 39275220
- PMCID: PMC11397412
- DOI: 10.3390/nu16172904
Adjusted versus Targeted Fortification in Extremely Low Birth Weight Preterm Infants: Fortin Study-A Randomized Clinical Trial
Abstract
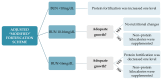
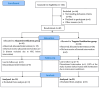
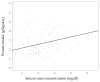
Fortified human milk is the first choice for preterm infants. Although individualized fortification is recommended, the optimal method for this population remains uncertain. We conducted a comparative study assessing the growth effects of adjusted (AF) and targeted fortification (TF) in extremely low birth weight (ELBW) infants. This single-center, randomized, controlled clinical trial was conducted at a tertiary neonatal unit in Spain. Eligible participants were premature infants with a birthweight of <1000 g exclusively fed with human milk. A total of 38 patients were enrolled, 15 of them randomized to AF group and 23 to TF group. AF was based on blood urea nitrogen (BUN) concentration and TF on human milk analysis. The primary outcome was weight gain velocity (g/kg/day). No significant differences were found in weight gain velocity at 28 days, at 36 weeks of postmenstrual age, at discharge, nor during the intervention. Protein intake was significantly higher in the AF group (5.02 g/kg/day vs. 4.48 g/kg/day, p = 0.001). No differences were found in the lipid, carbohydrate, and energy intake; in the weight z score change between the different time points; nor in the length and head circumference growth. Both AF and TF are comparable methods of fortification and provide the appropriate growth rate in ELBW infants.
Keywords: blood urea nitrogen; growth; human milk; human milk fortification; individualized fortification; neonatal nutrition; preterm infants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Arslanoglu S., Boquien C.Y., King C., Lamireau D., Tonetto P., Barnett D., Bertino E., Gaya A., Gebauer C., Grovslien A., et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019;7:76. doi: 10.3389/fped.2019.00076. - DOI - PMC - PubMed
-
- Vohr B.R., Poindexter B.B., Dusick A.M., McKinley L.T., Higgins R.D., Langer J.C., Poole W.K., Wright L.L., Bauer C.R., Meinzen-Derr J., et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120:e953–e959. doi: 10.1542/peds.2006-3227. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

