Promotion of a Mediterranean Diet Alters Constipation Symptoms and Fecal Calprotectin in People with Parkinson's Disease: A Randomized Controlled Trial
- PMID: 39275262
- PMCID: PMC11396875
- DOI: 10.3390/nu16172946
Promotion of a Mediterranean Diet Alters Constipation Symptoms and Fecal Calprotectin in People with Parkinson's Disease: A Randomized Controlled Trial
Abstract
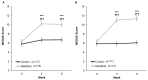
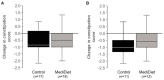
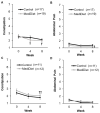
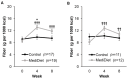
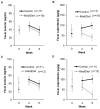
Parkinson's disease is associated with gastrointestinal (GI) dysfunction, including constipation symptoms and abnormal intestinal permeability and inflammation. A Mediterranean diet (MediDiet) may aid in disease management. This parallel, randomized, controlled trial in people with Parkinson's (PwP) and constipation symptoms compared a MediDiet against standard of care on change in constipation symptoms, dietary intake, and fecal zonulin and calprotectin concentrations as markers of intestinal permeability and inflammation, respectively. Participants were randomized to either standard of care for constipation (control; n = 17, 65.1 ± 2.2 years) or a MediDiet plus standard of care (n = 19, 68.8 ± 1.4 years) for 8 weeks. Constipation scores decreased with both interventions (p < 0.01), but changes from baseline were not different between groups (MediDiet, -0.5 [-1.0, 0]; control, -0.8 [-1.0, 0.2]; median [25th, 75th]; p = 0.60). The MediDiet group had a higher intake of dietary fiber at week 4 than the control group (13.1 ± 0.7 g/1000 kcal vs. 9.8 ± 0.7 g/1000 kcal; p < 0.001). No differences in fecal zonulin were observed between groups (p = 0.33); however, fecal calprotectin tended to be lower in the MediDiet group at week 8 (45.8 ± 15.1 µg/g vs. 93.9 ± 26.8 µg/g; p = 0.05). The MediDiet and standard interventions reduced constipation symptoms; however, the MediDiet provided additional benefit of increased dietary fiber intake and less intestinal inflammation.
Keywords: Mediterranean diet; Parkinson’s disease; constipation; inflammation; microbiota.
Conflict of interest statement
C.R. is now an employee of Abbott’s Nutrition Division, Columbus, OH, 43219, USA, and T.S. is employed by the Department of Neurology, Weill Institute for Neurosciences. University of California, San Francisco, San Francisco, CA, 94143-3126, USA; however, this work was completed while affiliated with the University of Florida. All other authors have no conflicts of interest to declare.
Figures
References
-
- Sampson T.R., Debelius J.W., Thron T., Janssen S., Shastri G.G., Ilhan Z.E., Challis C., Schretter C.E., Rocha S., Gradinaru V., et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2016;167:1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

