Indole-3-Butyric Acid, a Natural Auxin, Protects against Fenton Reaction-Induced Oxidative Damage in Porcine Thyroid
- PMID: 39275325
- PMCID: PMC11397436
- DOI: 10.3390/nu16173010
Indole-3-Butyric Acid, a Natural Auxin, Protects against Fenton Reaction-Induced Oxidative Damage in Porcine Thyroid
Abstract
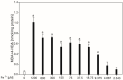
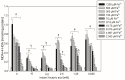
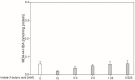
We present results on the potential protective antioxidant properties of indole-3-butyric acid. Indole-3-butyric acid is an indole derivative defined as an auxin and widely known as a plant growth regulator. It naturally occurs in Arabidopsis thaliana, which is applied as a model plant in genetic studies. Oxidative damage to membrane lipids (lipid peroxidation; LPO) in porcine thyroid homogenates was induced by Fenton reaction substrates (Fe2+ + H2O2). Iron (Fe2+) was used in very high concentrations of 1200, 600, 300, 150, 75, 37.5, 18.75, 9.375, 4.687, and 2.343 µM. Indole-3-butyric acid (10.0, 5.0, 2.5, 1.25, and 0.625 mM) was applied to check whether it prevents the above process. The LPO level, expressed as malondialdehyde + 4-hydroxyalkenals (MDA + 4-HDA) concentration, was measured spectrophotometrically. Expectedly, Fenton reaction substrates, in a Fe2+ concentration-dependent manner, increased LPO level, with the lowest effective concentration of iron being 9.375 µM. In the case of almost all concentrations of indole-3-butyric acid, this auxin has exhibited very promising antioxidant protection, with the most effective concentrations being 10.0 and 5.0 mM; however, as low concentrations of indole-3-butyric acid at 1.25 mM was still effective. Indole-3-butyric acid used alone did not change the basal level of LPO, which is a favourable effect. To summarise, indole-3-butyric acid has protective antioxidant properties against experimentally induced oxidative damage to membrane lipids in the thyroid, and this is for the first time documented in the literature. This compound can be considered a natural protective agent present in plants, which can serve as a dietary nutrient.
Keywords: Fenton reaction; antioxidant; indole-3-butyric acid; iron; lipid peroxidation; porcine thyroid; reactive oxygen species.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Melatonin reduces Fenton reaction-induced lipid peroxidation in porcine thyroid tissue.J Cell Biochem. 2003 Nov 1;90(4):806-11. doi: 10.1002/jcb.10689. J Cell Biochem. 2003. PMID: 14587035
-
Fenton Reaction-Induced Oxidative Damage to Membrane Lipids and Protective Effects of 17β-Estradiol in Porcine Ovary and Thyroid Homogenates.Int J Environ Res Public Health. 2020 Sep 18;17(18):6841. doi: 10.3390/ijerph17186841. Int J Environ Res Public Health. 2020. PMID: 32962175 Free PMC article.
-
Membrane lipids and nuclear DNA are differently susceptive to Fenton reaction substrates in porcine thyroid.Toxicol In Vitro. 2013 Feb;27(1):71-8. doi: 10.1016/j.tiv.2012.09.010. Epub 2012 Sep 26. Toxicol In Vitro. 2013. PMID: 23022769
-
Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators.Int J Mol Sci. 2019 Oct 8;20(19):4968. doi: 10.3390/ijms20194968. Int J Mol Sci. 2019. PMID: 31597407 Free PMC article. Review.
-
Lipid peroxidation and antioxidants as biomarkers of tissue damage.Clin Chem. 1995 Dec;41(12 Pt 2):1819-28. Clin Chem. 1995. PMID: 7497639 Review.
Cited by
-
Folic acid alleviates the negative effects of dexamethasone induced stress on production performance in Hyline Brown laying hens.Anim Nutr. 2024 Dec 14;20:54-65. doi: 10.1016/j.aninu.2024.11.011. eCollection 2025 Mar. Anim Nutr. 2024. PMID: 39949729 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

