Wearable Sensor-Based Assessments for Remotely Screening Early-Stage Parkinson's Disease
- PMID: 39275547
- PMCID: PMC11397844
- DOI: 10.3390/s24175637
Wearable Sensor-Based Assessments for Remotely Screening Early-Stage Parkinson's Disease
Abstract
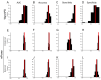
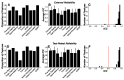
Prevalence estimates of Parkinson's disease (PD)-the fastest-growing neurodegenerative disease-are generally underestimated due to issues surrounding diagnostic accuracy, symptomatic undiagnosed cases, suboptimal prodromal monitoring, and limited screening access. Remotely monitored wearable devices and sensors provide precise, objective, and frequent measures of motor and non-motor symptoms. Here, we used consumer-grade wearable device and sensor data from the WATCH-PD study to develop a PD screening tool aimed at eliminating the gap between patient symptoms and diagnosis. Early-stage PD patients (n = 82) and age-matched comparison participants (n = 50) completed a multidomain assessment battery during a one-year longitudinal multicenter study. Using disease- and behavior-relevant feature engineering and multivariate machine learning modeling of early-stage PD status, we developed a highly accurate (92.3%), sensitive (90.0%), and specific (100%) random forest classification model (AUC = 0.92) that performed well across environmental and platform contexts. These findings provide robust support for further exploration of consumer-grade wearable devices and sensors for global population-wide PD screening and surveillance.
Keywords: Parkinson’s disease; digital biomarkers; early detection; feature engineering; gait analysis; mobile health technologies; phonation; remote monitoring; wearable sensors.
Conflict of interest statement
D. Anderson, J. Severson., A. Best, M. Merickel., D. Amato, B. Severson, S. Jezewski, S. Polyak, A. Keil, M. Kantartjis, and S. Johnson are employees of Clinical ink, who acquired the BrainBaseline Movement Disorders platform in 2021. E.R. Dorsey has received compensation for consulting services from Abbott, Abbvie, Acadia, Acorda, Bial-Biotech Investments, Inc., Biogen, Boehringer Ingelheim, California Pacific Medical Center, Caraway Therapeutics, Curasen Therapeutics, Denali Therapeutics, Eli Lilly, Genentech/Roche, Grand Rounds, Huntington Study Group, Informa Pharma Consulting, Karger Publications, LifeSciences Consultants, MCM Education, Mediflix, Medopad, Medrhythms, Merck, Michael J. Fox Foundation, NACCME, Neurocrine, NeuroDerm, NIH, Novartis, Origent Data Sciences, Otsuka, Physician’s Education Resource, Praxis, PRIME Education, Roach, Brown, McCarthy & Gruber, Sanofi, Seminal Healthcare, Spark, Springer Healthcare, Sunovion Pharma, Theravance, Voyager and WebMD; research support from Biosensics, Burroughs Wellcome Fund, CuraSen, Greater Rochester Health Foundation, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health, Patient-Centered Outcomes Research Institute, Pfizer, PhotoPharmics, Safra Foundation, and Wave Life Sciences; editorial services for Karger Publications; stock in Included Health and in Mediflix, and ownership interests in SemCap. J.L. Adams has received compensation for consulting services from VisualDx and the Huntington Study Group; and research support from Biogen, Biosensics, Huntington Study Group, Michael J. Fox Foundation, National Institutes of Health/National Institute of Neurological Disorders and Stroke, NeuroNext Network, and Safra Foundation. M.A. Kostrzebski holds stock in Apple, Inc. T. Kangarloo is an employee of and owns stock in Takeda Pharmaceuticals, Inc. J. Cosman Cosman is an employee of and owns stock in AbbVie Pharmaceuticals.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

