Cannabinol modulates the endocannabinoid system and shows TRPV1-mediated anti-inflammatory properties in human keratinocytes
- PMID: 39275884
- PMCID: PMC11681214
- DOI: 10.1002/biof.2122
Cannabinol modulates the endocannabinoid system and shows TRPV1-mediated anti-inflammatory properties in human keratinocytes
Abstract
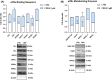
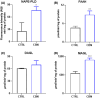
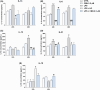
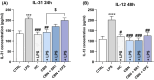
Cannabinol (CBN) is a secondary metabolite of cannabis whose beneficial activity on inflammatory diseases of human skin has attracted increasing attention. Here, we sought to investigate the possible modulation by CBN of the major elements of the endocannabinoid system (ECS), in both normal and lipopolysaccharide-inflamed human keratinocytes (HaCaT cells). CBN was found to increase the expression of cannabinoid receptor 1 (CB1) at gene level and that of vanilloid receptor 1 (TRPV1) at protein level, as well as their functional activity. In addition, CBN modulated the metabolism of anandamide (AEA) and 2-arachidonoylglicerol (2-AG), by increasing the activities of N-acyl phosphatidylethanolamines-specific phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH)-the biosynthetic and degradative enzyme of AEA-and that of monoacylglycerol lipase (MAGL), the hydrolytic enzyme of 2-AG. CBN also affected keratinocyte inflammation by reducing the release of pro-inflammatory interleukin (IL)-8, IL-12, and IL-31 and increasing the release of anti-inflammatory IL-10. Of note, the release of IL-31 was mediated by TRPV1. Finally, the mitogen-activated protein kinases (MAPK) signaling pathway was investigated in inflamed keratinocytes, demonstrating a specific modulation of glycogen synthase kinase 3β (GSK3β) upon treatment with CBN, in the presence or not of distinct ECS-directed drugs. Overall, these results demonstrate that CBN modulates distinct ECS elements and exerts anti-inflammatory effects-remarkably via TRPV1-in human keratinocytes, thus holding potential for both therapeutic and cosmetic purposes.
Keywords: cannabinol; cytokines; endocannabinoid system; keratinocytes; skin inflammation.
© 2024 The Author(s). BioFactors published by Wiley Periodicals LLC on behalf of International Union of Biochemistry and Molecular Biology.
Conflict of interest statement
The authors declare that the InMed Pharmaceuticals is commercializing cannabinoid‐based therapies. Eric Hsu is the Senior Vice President of this company; Salam Kadhim is a former Senior Scientist of Preclinical R&D, and Mauro Maccarrone is a Scientific Advisory Board member.
Figures



References
-
- Hanuš LO, Meyer SM, Muñoz E, Taglialatela‐Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33:1357–1392. - PubMed
-
- Pisanti S, Bifulco M. Medical Cannabis: a plurimillennial history of an evergreen. J Cell Physiol. 2019;234:8342–8351. - PubMed
-
- ElSohly MA, Radwan MM, Gul W, Chandra S, Galal A. Phytochemistry of Cannabis sativa L. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J, editors. Phytocannabinoids, progress in the chemistry of organic natural products. Cham: Springer International Publishing; 2017. p. 1–36. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

