Clinical and genetic delineation of autosomal recessive and dominant ACTL6B-related developmental brain disorders
- PMID: 39275948
- PMCID: PMC12042808
- DOI: 10.1016/j.gim.2024.101251
Clinical and genetic delineation of autosomal recessive and dominant ACTL6B-related developmental brain disorders
Abstract
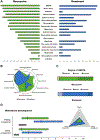
Purpose: This study aims to comprehensively delineate the phenotypic spectrum of ACTL6B-related disorders, previously associated with both autosomal recessive and autosomal dominant neurodevelopmental disorders. Molecularly, the role of the nucleolar protein ACTL6B in contributing to the disease has remained unclear.
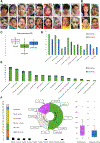
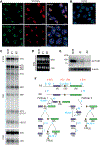
Methods: We identified 105 affected individuals, including 39 previously reported cases, and systematically analyzed detailed clinical and genetic data for all individuals. Additionally, we conducted knockdown experiments in neuronal cells to investigate the role of ACTL6B in ribosome biogenesis.
Results: Biallelic variants in ACTL6B are associated with severe-to-profound global developmental delay/intellectual disability, infantile intractable seizures, absent speech, autistic features, dystonia, and increased lethality. De novo monoallelic variants result in moderate-to-severe global developmental delay/intellectual disability, absent speech, and autistic features, whereas seizures and dystonia were less frequently observed. Dysmorphic facial features and brain abnormalities, including hypoplastic corpus callosum, and parenchymal volume loss/atrophy, are common findings in both groups. We reveal that in the nucleolus, ACTL6B plays a crucial role in ribosome biogenesis, particularly in pre-rRNA processing.
Conclusion: This study provides a comprehensive characterization of the clinical spectrum of both autosomal recessive and dominant forms of ACTL6B-associated disorders. It offers a comparative analysis of their respective phenotypes provides a plausible molecular explanation and suggests their inclusion within the expanding category of "ribosomopathies."
Keywords: ACTL6B; Autism; BAFopathies; Epileptic-dyskinetic encephalopathy; Ribosomopathies.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest Sureni V. Mullegama and Amber Begtrup are employees of GeneDx. Lee Hane is an employee at 3billion. Christian Beetz is an employee at Centogene. All other authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

