[Therapeutic mechanism of Cynanchum wilfordii for ulcerative colitis: an analysis using UPLC-QE-MS, network pharmacology and metabolomics]
- PMID: 39276044
- PMCID: PMC11378042
- DOI: 10.12122/j.issn.1673-4254.2024.08.07
[Therapeutic mechanism of Cynanchum wilfordii for ulcerative colitis: an analysis using UPLC-QE-MS, network pharmacology and metabolomics]
Abstract
Objective: To explore the targets and pathways of Cynanchum wilfordii for treatment of ulcerative colitis (UC).
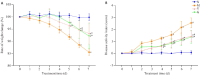
Methods: UPLC-QE-MS was used to identify the components of Cynanchum wilfordii ethanol extract, and their targets were screened using public databases for construction of the core protein-protein interaction (PPI) network and GO and KEGG enrichment analyses. Forty male C57 mice were randomized into normal control group, model group, mesalazine group and Cynanchum wilfordii group (n=10), and in the latter 3 groups, mouse UC models were established by treatment with 2.5% DSS and the latter 2 groups drug interventions by gavage. The therapeutic effect was evaluated by recording body weight changes and DAI score. Pathological changes of the colon tissue were observed with HE and AB-PAS staining, and JAK2 and STAT3 protein expressions were detected with Western blotting. The metabolites and metabolic pathways were identified by metabonomics analysis.
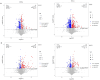
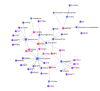
Results: We identified 240 chemical components in Cynanchum wilfordii alcoholic extracts, including 19 steroids. A total of 177 Cynanchum wilfordii targets, 5406 UC genes, and 117 intersection genes were obtained. JAK2 and STAT3 were the core targets and significantly enriched in lipid and atherosclerosis pathways. Cynanchum wilfordii treatment significantly increased the body weight and decreased DAI score of UC mice (P < 0.05), alleviated intestinal pathologies, and decreased JAK2 and STAT3 protein expressions in the colon tissues. Most of the 83 intersecting differential metabolites between the control, model and Cynanchum wilfordii groups were identified as glycerophospholipids, arachidonic acid, and amino acids involving glycerophospholipid metabolism and other pathways. Correlation analysis suggested that the core targets of Cynanchum wilfordii for UC participated in regulation of the metabolites.
Conclusion: Cynanchum wilfordii alleviates lipid and amino acid metabolism disorders to lessen UC in mice by regulating the core targets including JAK2 and STAT3 and the levels of endogenous metabolites.
目的: 基于UPLC-QE-MS与网络药理学挖掘隔山消防治溃疡性结肠炎(UC)的靶点及通路,并结合代谢组学技术探讨作用机制。
方法: 采用UPLC-QE-MS技术鉴定隔山消醇提物化学成分,基于Swiss Target Prediction、GeneCards、Pubchem等数据库筛选相应靶点,获取核心PPI,进行GO、KEGG富集分析。将40只雄性C57小鼠随机分为正常组、模型组、美沙拉嗪组(0.2 g/kg)、隔山消组(2.28 g/kg),每组10只,除正常组外,其余各组自由饮用2.5% DSS诱导UC模型,造模期间给药组给予药物灌胃干预。通过体质量变化率、DAI得分评价治疗效果;HE及AB-PAS染色观察结肠组织病理变化;Western blotting技术检测JAK2、STAT3蛋白水平;代谢组学技术鉴别差异代谢物并挖掘代谢通路。
结果: 鉴定出隔山消醇提物化学成分240个,其中甾体类(高含量)19个,得到隔山消(甾体类)靶点177个,UC基因5406个,隔山消与UC交集基因117个,JAK2、STAT3等为核心PPI,在脂质与动脉粥样硬化等通路富集显著。动物实验结果显示,经隔山消治疗后,小鼠体质量变化率上升、DAI评分显著下降(P<0.05),肠组织病理改变明显缓解,JAK2、STAT3蛋白水平显著降低(P<0.05)。鉴定出正常组、模型组及隔山消组之间交集差异代谢物83个,以甘油磷脂、类花生酸、氨基酸成分为主,与甘油磷脂代谢等通路相关。整合分析显示隔山消治疗UC的核心靶点参与了代谢物的调节。
结论: 隔山消可通过调节JAK2、STAT3等核心靶点表达及内源性代谢物水平来缓解脂质及氨基酸代谢紊乱,发挥治疗UC的作用。
Keywords: Cynanchum wilfordii (Maxim.) Hook. F; UPLC-QE-MS; metabolomics; steroids; ulcerative colitis.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
