[Platelet-specific Rictor knockout inhibits platelet production and activation and reduces thrombosis in mice]
- PMID: 39276057
- PMCID: PMC11378050
- DOI: 10.12122/j.issn.1673-4254.2024.08.20
[Platelet-specific Rictor knockout inhibits platelet production and activation and reduces thrombosis in mice]
Abstract
Objective: To investigate the effects of platelet-specific Rictor knockout on platelet activation and thrombus formation in mice.
Methods: PF4-Cre and Rictorfl/fl transgenic mice were crossed to obtain platelet-specific Rictor knockout (Rictor-KO) mice and wild-type mice (n=65), whose expression levels of Rictor, protein kinase B (AKT) and p-AKT were detected using Western blotting. Platelet counts of the mice were determined using routine blood tests, and hemostatic function was assessed by tail vein hemorrhage test. Venous thrombosis models were established in the mice to evaluate the effect of Rictor knockout on thrombosis. Platelet aggregation induced by ADP and thrombin was observed in Rictor-KO and wild-type mice, and flow cytometry was used to analyze the expression levels of integrin αIIbβ3 and CD62P in resting and activated platelets. Plasma PF4 levels were determined with ELISA. Megakaryocytes from Rictor-KO and wild-type mice were incubated by vWF immunohistochemical antibody and APC-CD41 antibody to detect the number and ploidy of megakaryocytes, respectively. Platelet elongation on collagen surface was observed with scanning electron microscopy.
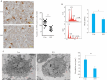
Results: Compared with the wild-type mice, Rictor-KO mice showed significantly decreased AKT phosphorylation, decreased platelet production, reduced thrombosis, and decreased platelet activation in response to ADP and thrombin stimulation. The Rictor-KO mice also showed lowered expression level of P-selectin protein and activation of integrin αIIbβ3 with suppression of platelet extension, reduced plasma PF4 level and decreased number of megakaryocytes in the bone marrow. The ploidy of megakaryocytes and the mean area of proplatelets were both significantly decreased in Rictor-KO mice.
Conclusion: Platelet-specific Rictor knockout inhibits platelet generation and activation to result in decreased thrombus formation in mice, suggesting the potential of mTORC2 activity inhibition as an efficient antithrombotic strategy.
目的: 探究Rictor对血小板活化和血栓形成的影响。
方法: 利用PF4-Cre、Rictorfl/fl转基因小鼠,构建血小板特异性Rictor敲除小鼠和野生型小鼠,分别设为敲除组和对照组(总实验小鼠n=65),用于后续实验。Western blotting检测小鼠Rictor蛋白、蛋白激酶B(AKT)、p-AKT的表达水平。血常规检测敲除和对照组小鼠血小板数目。尾静脉出血实验检测敲除和对照小鼠止血功能。构建静脉血栓模型检测Rictor敲除对血栓形成的影响。使用ADP和凝血酶刺激血小板,检测Rictor敲除对血小板聚集的影响。流式细胞术分析敲除组和对照组血小板在静息态和活化态整合素αIIbβ3与CD62P的表达水平。检测血浆中PF4水平。vWF免疫组化标记巨核细胞。用APC-CD41抗体孵育敲除和对照小鼠的巨核细胞,流式细胞术检测细胞倍体。扫描电镜检测敲除和对照组血小板在胶原表面的伸展变化。
结果: 与对照小鼠相比,血小板Rictor特异性敲除小鼠AKT磷酸化降低(P<0.001),血小板生成减少(P<0.05),血栓形成受到抑制(P<0.05),血小板对ADP和凝血酶刺激的活化水平降低(P<0.01),P-选择素蛋白表达水平(P<0.05)、整合素αIIbβ3活化程度(P<0.01)受到抑制,血小板伸展受到抑制(P<0.01),PF4的血浆含量降低(P<0.05),骨髓中巨核细胞数量减少(P<0.01),倍体降低(P<0.05),前血小板平均面积减少(P<0.01)。
结论: 血小板特异性敲除Rictor抑制血小板生成和活化,进而减少血栓形成。抑制mTORC2活性可能是一种潜在的抗血栓靶标。
Keywords: Rictor; platelets; thrombosis.
Figures



Similar articles
-
A novel role for thioredoxin-related transmembrane protein TMX4 in platelet activation and thrombus formation.J Thromb Haemost. 2025 Jan;23(1):277-292. doi: 10.1016/j.jtha.2024.09.007. Epub 2024 Sep 20. J Thromb Haemost. 2025. PMID: 39307246
-
Myosin light chain 6 (Myl6) interacts with kindlin-3 and is required to support integrin αIIbβ3 activation in platelets in mice.J Thromb Haemost. 2024 Jul;22(7):2009-2017. doi: 10.1016/j.jtha.2024.01.007. Epub 2024 Jan 22. J Thromb Haemost. 2024. PMID: 38266679 Free PMC article.
-
Inflammatory cytokine TSLP stimulates platelet secretion and potentiates platelet aggregation via a TSLPR-dependent PI3K/Akt signaling pathway.Cell Physiol Biochem. 2015;35(1):160-74. doi: 10.1159/000369684. Epub 2015 Jan 2. Cell Physiol Biochem. 2015. PMID: 25591759
-
Reversible Platelet Integrin αIIbβ3 Activation and Thrombus Instability.Int J Mol Sci. 2022 Oct 19;23(20):12512. doi: 10.3390/ijms232012512. Int J Mol Sci. 2022. PMID: 36293367 Free PMC article. Review.
-
Emerging Technologies for Understanding Platelet Diversity.Arterioscler Thromb Vasc Biol. 2022 May;42(5):540-552. doi: 10.1161/ATVBAHA.121.317092. Epub 2022 Mar 17. Arterioscler Thromb Vasc Biol. 2022. PMID: 35296153 Review.
References
-
- Næss IA, Christiansen SC, Romundstad P, et al. . Incidence and mortality of venous thrombosis: a population-based study[J]. J Thromb Haemost, 2007, 5(4): 692-9. - PubMed
-
- Camerer E, Kolstø AB, Prydz H. Cell biology of tissue factor, the principal initiator of blood coagulation[J]. Thromb Res, 1996, 81(1): 1-41. - PubMed
-
- Rojnuckarin P, Kaushansky K. Actin reorganization and proplatelet formation in murine megakaryocytes: the role of protein kinase calpha[J]. Blood, 2001, 97(1): 154-61. - PubMed
-
- Cramer EM, Norol F, Guichard J, et al. . Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand[J]. Blood, 1997, 89(7): 2336-46. - PubMed
-
- Flaumenhaft R. A new story ARC for α‑granule formation[J]. Blood, 2015, 126(2): 123-4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
