Wetting Preference of Silica Surfaces in the Context of Underground Hydrogen Storage: A Molecular Dynamics Perspective
- PMID: 39276104
- PMCID: PMC11447897
- DOI: 10.1021/acs.langmuir.4c02311
Wetting Preference of Silica Surfaces in the Context of Underground Hydrogen Storage: A Molecular Dynamics Perspective
Abstract
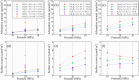
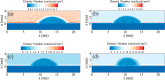
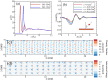
The growing interest in large-scale underground hydrogen (H2) storage (UHS) emphasizes the need for a comprehensive understanding of the fundamental characteristics of subsurface environments. The wetting preference of subsurface rock is a crucial parameter influencing the H2 flow behavior during storage and withdrawal processes. In this study, we utilized molecular dynamics simulation to evaluate the wetting preference of the silica surface in subsurface hydrogen systems, with the aim of addressing disparities observed in experimental results. We conducted an initial comprehensive assessment of potential models, comparing the wettability of five common silica surfaces with different surface morphologies and hydroxyl densities in CO2-H2/water/silica systems against experimental data. After introducing the INTERFACE force field as the most accurate potential model for the silica surface, we evaluated the wetting behavior of the α-quartz (101) surface with a hydroxyl density of 5.9 number/nm2 under the impact of actual geological storage conditions (333-413 K and 10-30 MPa), the coexistence of cushion gases (i.e., CO2, CH4, and N2) at various mole fractions, and pH levels ranging from 2 to 11 characterized through considering the negative charges of 0 to -0.12 C/m2 via deprotonation of silanol on the silica surface. Our results indicate that neither pressure nor temperature has a significant impact on the wetness of the silica in the case of pure H2 (single component UHS operations). However, when CO2 coexists with H2, especially at higher mole fractions, an increase in pressure and a decrease in temperature lead to higher contact angles. Moreover, when the mole fraction of cushion gas ranges from 0 to 1, the contact angle increases 20, 9.5, and 4.5° for CO2, CH4, and N2, respectively, on the neutral silica substrate. Interestingly, at higher pH conditions where the silica surface carries a negative charge, the contact angle considerably reduces where surface charges of -0.03 and -0.06 C/m2 result in an average reduction of 20 and 80% in the contact angle, respectively. More importantly, at a pH of ∼11 (-0.12 C/m2), a 0° contact angle is observed for the silica surface under all temperatures, pressures, types of cushion gases, and varying mole fractions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Seo S.-K.; Yun D.-Y.; Lee C.-J. Design and optimization of a hydrogen supply chain using a centralized storage model. Appl. Energy 2020, 262, 114452. 10.1016/j.apenergy.2019.114452. - DOI
-
- Gabrielli P.; Poluzzi A.; Kramer G. J.; Spiers C.; Mazzotti M.; Gazzani M. Seasonal energy storage for zero-emissions multi-energy systems via underground hydrogen storage. Renewable Sustainable Energy Rev. 2020, 121, 109629. 10.1016/j.rser.2019.109629. - DOI
-
- Acar C.; Dincer I. Review and evaluation of hydrogen production options for better environment. J. Cleaner Prod. 2019, 218, 835–849. 10.1016/j.jclepro.2019.02.046. - DOI
-
- Epelle E. I.; Obande W.; Udourioh G. A.; Afolabi I. C.; Desongu K. S.; Orivri U.; Gunes B.; Okolie J. A. Perspectives and prospects of underground hydrogen storage and natural hydrogen. Sustainable Energy Fuels 2022, 6, 3324–3343. 10.1039/D2SE00618A. - DOI
-
- Tarkowski R. Underground hydrogen storage: Characteristics and prospects. Renewable Sustainable Energy Rev. 2019, 105, 86–94. 10.1016/j.rser.2019.01.051. - DOI
LinkOut - more resources
Full Text Sources

