Histopathological response to chemotherapy and survival of mucinous type gastric cancer
- PMID: 39276158
- PMCID: PMC11807439
- DOI: 10.1093/jnci/djae227
Histopathological response to chemotherapy and survival of mucinous type gastric cancer
Abstract
Background: Data on the clinicopathological characteristics of mucinous gastric cancer (muc-GC) are limited. This study compares the clinical outcome and response to chemotherapy between patients with resectable muc-GC, intestinal (int-GC), and diffuse (dif-GC) gastric cancer.
Methods: Patients from the D1/D2 study or the CRITICS trial were included in exploratory surgery-alone (SAtest) or chemotherapy test (CTtest) cohorts. Real-world data from the Netherlands Cancer Registry on patients treated between with surgery alone (SAvalidation) and receiving preoperative chemotherapy with or without postoperative treatment (CTvalidation) were used for validation. Histopathological subtypes were extracted from pathology reports filed in the Dutch Pathology Registry and correlated with tumor regression grade (TRG) and relative survival (RS).
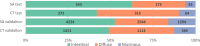
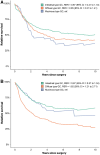
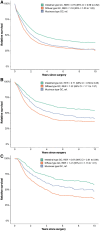
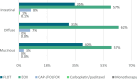
Results: In the SAtest (n = 549) and SAvalidation (n = 8062) cohorts, muc-GC patients had a 5-year RS of 39% and 31%, similar to or slightly better than dif-GC (43% and 29%, P = .52 and P = .011), but worse than int-GC (55% and 42%, P = .11 and P < .001). In the CTtest (n = 651) and CTvalidation (n = 2889) cohorts, muc-GC showed favorable TRG (38% and 44% (near-) complete response) compared with int-GC (26% and 35%) and dif-GC (10% and 28%, P < .001 and P = .005). The 5-year RS in the CTtest and CTvalidation cohorts for muc-GC (53% and 48%) and int-GC (58% and 59%) was significantly better compared with dif-GC (35% and 38%, P = .004 and P < .001).
Conclusion: Recognizing and incorporating muc-GC into treatment decision-making of resectable GC can lead to more personalized and effective approaches, given its favorable response to preoperative chemotherapy in relation to int-GC and dif-GC and its favorable prognostic outcomes in relation to dif-GC.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
LVB received personal fees from Ipsen, Medtalks, BMS, Servier, and Travel Congress Management; MIvBH received consultancy fees from Viatris, Johnson & Johnson, Alesi Surgical, BBraun, Stryker, and Medtronic (paid to institute); GAPN received compensation for Clinical Immersions and Educational Meetings from Medtronic; RHAV received grants from BMS and consultancy fees from Daiichi-Sankyo (paid to institute). NCTVG received consultancy fees from BMS, Medtalks, Diaceutics, Astellas, and MSD. All remaining authors have declared no conflicts of interest.
Figures
References
-
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64(1):31-49. - PubMed
-
- Stiekema J, Cats A, Kuijpers A, et al. Surgical treatment results of intestinal and diffuse type gastric cancer: implications for a differentiated therapeutic approach? Eur J Surg Oncol. 2013;39(7):686-693. - PubMed
-
- Al-Batran S-E, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697-1708. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

