Eiken syndrome with parathyroid hormone resistance due to a novel parathyroid hormone receptor type 1 mutation: clinical features and functional analysis
- PMID: 39276366
- PMCID: PMC11523111
- DOI: 10.1093/jbmr/zjae148
Eiken syndrome with parathyroid hormone resistance due to a novel parathyroid hormone receptor type 1 mutation: clinical features and functional analysis
Abstract
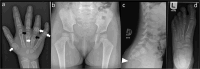
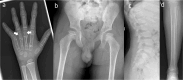
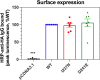
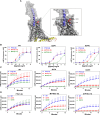
We report on 2 patients of East African ancestry with the same novel homozygous variant in the parathyroid hormone receptor type 1 (PTH1R). Both patients shared skeletal features, including brachydactyly, extensive metacarpal pseudo-epiphyses, elongated cone-shaped epiphyses, ischiopubic hypoplasia, and deficient sacral ossification, suggestive of Eiken syndrome. Strikingly, both patients exhibited clinically manifest parathyroid hormone (PTH) resistance with hypocalcemia and elevated serum phosphate levels. These laboratory and clinical abnormalities initially suggested pseudohypoparathyroidism, which is typically associated with GNAS abnormalities. In both patients, however, a homozygous novel PTH1R variant was identified (c.710 T > A; p.IIe237Asn, p.I237N) that is located in the second transmembrane helical domain. Previously, others have reported a patient with a nearby PTH1R mutation (D241E) who presented with similar clinical features (eg, delayed bone mineralization as well as clinical PTH resistance). Functional analysis of the effects of both novel PTH1R variants (I237N- and D241E-PTH1R) in HEK293 reporter cells transfected with plasmid DNA encoding the wild-type or mutant PTH1Rs demonstrated increased basal cAMP signaling for both variants, with relative blunting of responses to both PTH and PTH-related peptide (PTHrP) ligands. The clinical presentation of PTH resistance and delayed bone mineralization combined with the functional properties of the mutant PTH1Rs suggest that this form of Eiken syndrome results from alterations in PTH1R-mediated signaling in response to both canonical ligands, PTH and PTHrP.
Keywords: Eiken syndrome; GPCR disease variants; PTH1R; calcium homeostasis; delayed ossification; parathyroid hormone resistance.
Plain language summary
Eiken syndrome is rare genetic disorder of skeletal development, 7 due to alterations in the gene for parathyroid hormone receptor type 1 (PTH1R). This receptor can bind 2 hormones: parathyroid hormone (PTH), the body’s main regulator of the level of calcium in blood, and PTH-related peptide (PTHrP), that regulates bone development. We report 2 new cases of Eiken syndrome sharing the same not previously reported change in PTH1R. The patients had typical findings in the skeleton reported in previous cases, but with some variation in the features. Unlike previously reported cases, the 2 patients we describe had low blood calcium levels causing symptoms. We wanted to explore how this new mutation affects the function of the receptor, particularly how it might affect the signals generated when the receptor binds to its 2 t hormones, PTH and PTHrP. We genetically reprogrammed a cell line with the new mutation, and tested those cells’ responses to stimulation by the hormones. We showed that the altered receptor appears unable to bind both hormones in a stable fashion, explaining why the patients showed changes both in the skeleton (due to altered PTHrP signaling) and in the blood level of calcium (due to altered PTH signaling).
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

