Oncolytic adenovirus MEM-288 encoding membrane-stable CD40L and IFNβ induces an anti-tumor immune response in high grade serous ovarian cancer
- PMID: 39276533
- PMCID: PMC11417341
- DOI: 10.1016/j.neo.2024.101056
Oncolytic adenovirus MEM-288 encoding membrane-stable CD40L and IFNβ induces an anti-tumor immune response in high grade serous ovarian cancer
Abstract
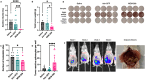
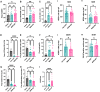
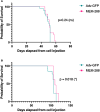
Single agent immune checkpoint inhibitors have been ineffective for patients with advanced stage and recurrent high grade serous ovarian cancer (HGSOC). Using pre-clinical models of HGSOC, we evaluated the anti-tumor and immune stimulatory effects of an oncolytic adenovirus, MEM-288. This conditionally replicative virus encodes a modified membrane stable CD40L and IFNβ. We demonstrated this virus successfully infects HGSOC cell lines and primary human ascites samples in vitro. We evaluated the anti-tumor and immunostimulatory activity in vivo in immune competent mouse models. Intraperitoneal delivery of MEM-288 decreased ascites and solid tumor burden compared to controls, and treatment generated a systemic anti-tumor immune response. The tumor microenvironment had a higher proportion of anti-tumor macrophages and decreased markers of angiogenesis. MEM-288 is a promising immunotherapy agent in HGSOC, with further pre-clinical studies required to understand the mechanism of action in the peritoneal microenvironment and clinical activity in combination with other therapies.
Keywords: Ascites; ELISA; ELISPOT; Immunotherapy; Intraperitoneal; Microenvironment; Oncolytic virus; Ovarian cancer.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Mark Cantwell is an employee, stock owner of Memgen, Inc and has a patent on MEM-288.
Figures

References
-
- Cancer Stat Facts: Ovarian Cancer. https://seer.cancer.gov/statfacts/html/ovary.html.
-
- Walker J.L., Brady M.F., Wenzel L., Fleming G.F., Huang H.Q., DiSilvestro P.A., Fujiwara K., Alberts D.S., Zheng W., Tewari K.S., et al. Randomized trial of intravenous versus intraperitoneal chemotherapy plus bevacizumab in advanced ovarian carcinoma: an NRG Oncology/Gynecologic oncology group study. J. Clin. Oncol. 2019;37:1380–1390. doi: 10.1200/JCO.18.01568. - DOI - PMC - PubMed
-
- Van Driel W.J., Koole S.N., Sikorska K., Schagen Van Leeuwen J.H., Schreuder H.W.R., Hermans R.H.M., De Hingh I.H.J.T., Van Der Velden J., Arts H.J., Massuger L.F.A.G., et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. New Engl. J. Med. 2018;378:230–240. doi: 10.1056/nejmoa1708618. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

