Gene therapy for the leukodystrophies: From preclinical animal studies to clinical trials
- PMID: 39276676
- PMCID: PMC11418141
- DOI: 10.1016/j.neurot.2024.e00443
Gene therapy for the leukodystrophies: From preclinical animal studies to clinical trials
Abstract
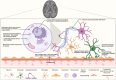
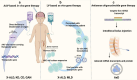
Leukodystrophies are progressive single gene disorders affecting the white matter of the brain. Several gene therapy trials are in progress to address the urgent unmet need for this patient population. We performed a comprehensive literature review of all gene therapy clinical trials listed in www.clinicaltrials.gov through August 2024, and the relevant preclinical studies that enabled clinical translation. Of the approximately 50 leukodystrophies described to date, only eight have existing gene therapy clinical trials: metachromatic leukodystrophy, X-linked adrenoleukodystrophy, globoid cell leukodystrophy, Canavan disease, giant axonal neuropathy, GM2 gangliosidoses, Alexander disease and Pelizaeus-Merzbacher disease. What led to the emergence of gene therapy trials for these specific disorders? What preclinical data or disease context was enabling? For each of these eight disorders, we first describe its pathophysiology and clinical presentation. We discuss the impact of gene therapy delivery route, targeted cell type, delivery modality, dosage, and timing on therapeutic efficacy. We note that use of allogeneic hematopoietic stem cell transplantation in some leukodystrophies allowed for an accelerated path to clinic even in the absence of available animal models. In other leukodystrophies, small and large animal model studies enabled clinical translation of experimental gene therapies. Human clinical trials for the leukodystrophies include ex vivo lentiviral gene delivery, in vivo AAV-mediated gene delivery, and intrathecal antisense oligonucleotide approaches. We outline adverse events associated with each modality focusing specifically on genotoxicity and immunotoxicity. We review monitoring and management of events related to insertional mutagenesis and immune responses. The data presented in this review show that gene therapy, while promising, requires systematic monitoring to account for the precarious disease biology and the adverse events associated with new technology.
Keywords: Adeno-associated viral vector; Antisense oligonucleotide; Gene therapy; Lentiviral vector; Leukodystrophies.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest F.S. Eichler receives research support from NINDS (R01NS072446, R01NS082331, U54NS115052), institutional contracts from ASPA Therapeutics, Sanofi, Ionis, bluebird bio and Minoryx Therapeutics, and performs personal consulting for Sanofi, ASPA Therapeutics, UpToDate, Leal, Takeda, Atlas Venture, Acadia Pharmaceuticals and SwanBio Therapeutics.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

