Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy
- PMID: 39276774
- PMCID: PMC11623344
- DOI: 10.1016/j.cell.2024.08.029
Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy
Abstract
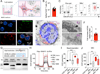
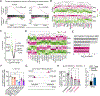
Mitochondrial loss and dysfunction drive T cell exhaustion, representing major barriers to successful T cell-based immunotherapies. Here, we describe an innovative platform to supply exogenous mitochondria to T cells, overcoming these limitations. We found that bone marrow stromal cells establish nanotubular connections with T cells and leverage these intercellular highways to transplant stromal cell mitochondria into CD8+ T cells. Optimal mitochondrial transfer required Talin 2 on both donor and recipient cells. CD8+ T cells with donated mitochondria displayed enhanced mitochondrial respiration and spare respiratory capacity. When transferred into tumor-bearing hosts, these supercharged T cells expanded more robustly, infiltrated the tumor more efficiently, and exhibited fewer signs of exhaustion compared with T cells that did not take up mitochondria. As a result, mitochondria-boosted CD8+ T cells mediated superior antitumor responses, prolonging animal survival. These findings establish intercellular mitochondrial transfer as a prototype of organelle medicine, opening avenues to next-generation cell therapies.
Keywords: CAR T therapy; CD8(+) T cells; TCR-T therapy; TIL therapy; Talin 2; bone marrow stromal cells; cancer immunotherapy; immune metabolism; mitochondrial transfer; nanotubes.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests J.G.B., T.S., J.F., S.S., and L.G. have a patent application for the use of mitochondrial transfer technology in cancer immunotherapies. P.B. and S.G. have an employee relationship and have stock in AstraZeneca. B.W.H. has an employee relationship and has stock in Genmab. L.G. has consulting agreements with Lyell Immunopharma, Instil Bio, and Advaxis. L.G. is on the scientific advisory board of Poseida Therapeutics and Kiromic and a stockholder of Poseida Therapeutics. M.I. participates in advisory boards/consultancies for Gilead Sciences, Third Rock Ventures, Antios Therapeutics, Asher Biotherapeutics, GentiBio, Clexio Biosciences, Sybilla, and BlueJay Therapeutics. J.F. has an employee relationship and has stock in Lyell Immunopharma. S.S. is a founder and owns equity in Vyome Therapeutics Inc. and Alyssum Therapeutics Inc.
Figures





References
-
- Scharping NE, Rivadeneira DB, Menk AV, Vignali PDA, Ford BR, Rittenhouse NL, Peralta R, Wang Y, Wang Y, DePeaux K, et al. (2021). Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nature Immunology 22, 205–215. 10.1038/s41590-020-00834-9. - DOI - PMC - PubMed
-
- Soto-Heredero G, Desdín-Micó G, and Mittelbrunn M. (2021). Mitochondrial dysfunction defines T cell exhaustion. Cell Metabolism 33, 470–472. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

