Dual client binding sites in the ATP-independent chaperone SurA
- PMID: 39277579
- PMCID: PMC11401910
- DOI: 10.1038/s41467-024-52021-1
Dual client binding sites in the ATP-independent chaperone SurA
Abstract
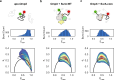
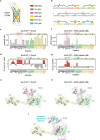
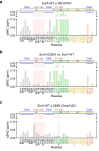
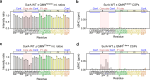
The ATP-independent chaperone SurA protects unfolded outer membrane proteins (OMPs) from aggregation in the periplasm of Gram-negative bacteria, and delivers them to the β-barrel assembly machinery (BAM) for folding into the outer membrane (OM). Precisely how SurA recognises and binds its different OMP clients remains unclear. Escherichia coli SurA comprises three domains: a core and two PPIase domains (P1 and P2). Here, by combining methyl-TROSY NMR, single-molecule Förster resonance energy transfer (smFRET), and bioinformatics analyses we show that SurA client binding is mediated by two binding hotspots in the core and P1 domains. These interactions are driven by aromatic-rich motifs in the client proteins, leading to SurA core/P1 domain rearrangements and expansion of clients from collapsed, non-native states. We demonstrate that the core domain is key to OMP expansion by SurA, and uncover a role for SurA PPIase domains in limiting the extent of expansion. The results reveal insights into SurA-OMP recognition and the mechanism of activation for an ATP-independent chaperone, and suggest a route to targeting the functions of a chaperone key to bacterial virulence and OM integrity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

