Population suppression by release of insects carrying a dominant sterile homing gene drive targeting doublesex in Drosophila
- PMID: 39277611
- PMCID: PMC11401859
- DOI: 10.1038/s41467-024-52473-5
Population suppression by release of insects carrying a dominant sterile homing gene drive targeting doublesex in Drosophila
Abstract
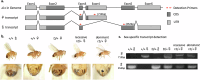
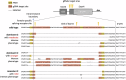
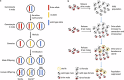
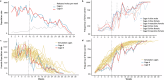
CRISPR homing gene drives can suppress pest populations by targeting female fertility genes, converting wild-type alleles into drive alleles in the germline of drive heterozygotes. fsRIDL (female-specific Release of Insects carrying a Dominant Lethal) is a self-limiting population suppression strategy involving continual release of transgenic males carrying female lethal alleles. Here, we propose an improved pest suppression system called "Release of Insects carrying a Dominant-sterile Drive" (RIDD), combining performance characteristics of homing drive and fsRIDL. We construct a split RIDD system in Drosophila melanogaster by creating a 3-gRNA drive disrupting the doublesex female exon. Drive alleles bias their inheritance in males, while drive alleles and resistance alleles formed by end-joining cause dominant female sterility. Weekly releases of RIDD males progressively suppressed and eventually eliminated cage populations. Modeling shows that RIDD is substantially stronger than SIT and fsRIDL. RIDD is also self-limiting, potentially allowing targeted population suppression.
© 2024. The Author(s).
Conflict of interest statement
The authors have declared no competing interest.
Figures

Similar articles
-
Population suppression with dominant female-lethal alleles is boosted by homing gene drive.BMC Biol. 2024 Sep 11;22(1):201. doi: 10.1186/s12915-024-02004-x. BMC Biol. 2024. PMID: 39256812 Free PMC article.
-
Germline Cas9 promoters with improved performance for homing gene drive.Nat Commun. 2024 May 29;15(1):4560. doi: 10.1038/s41467-024-48874-1. Nat Commun. 2024. PMID: 38811556 Free PMC article.
-
A homing suppression gene drive with multiplexed gRNAs maintains high drive conversion efficiency and avoids functional resistance alleles.G3 (Bethesda). 2022 May 30;12(6):jkac081. doi: 10.1093/g3journal/jkac081. G3 (Bethesda). 2022. PMID: 35394026 Free PMC article.
-
Manipulating Insect Sex Determination Pathways for Genetic Pest Management: Opportunities and Challenges.Front Bioeng Biotechnol. 2022 Jun 28;10:867851. doi: 10.3389/fbioe.2022.867851. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35837548 Free PMC article. Review.
-
Biotechnology-enhanced genetic controls of the global pest Drosophila suzukii.Trends Biotechnol. 2025 Apr;43(4):826-837. doi: 10.1016/j.tibtech.2024.09.005. Epub 2024 Sep 25. Trends Biotechnol. 2025. PMID: 39327106 Review.
Cited by
-
Advancements and Future Prospects of CRISPR-Cas-Based Population Replacement Strategies in Insect Pest Management.Insects. 2024 Aug 30;15(9):653. doi: 10.3390/insects15090653. Insects. 2024. PMID: 39336621 Free PMC article. Review.
-
Engineering drive-selection balance for localized population suppression with neutral dynamics.Proc Natl Acad Sci U S A. 2025 Feb 11;122(6):e2414207122. doi: 10.1073/pnas.2414207122. Epub 2025 Feb 4. Proc Natl Acad Sci U S A. 2025. PMID: 39903106 Free PMC article.
-
A Y chromosome-linked genome editor for efficient population suppression in the malaria vector Anopheles gambiae.Nat Commun. 2025 Jan 2;16(1):206. doi: 10.1038/s41467-024-55391-8. Nat Commun. 2025. PMID: 39747012 Free PMC article.
-
Gene drive-based population suppression in the malaria vector Anopheles stephensi.Nat Commun. 2025 Jan 24;16(1):1007. doi: 10.1038/s41467-025-56290-2. Nat Commun. 2025. PMID: 39856077 Free PMC article.
-
A comprehensive review of biological and genetic control approaches for leishmaniasis vector sand flies; emphasis towards promoting tools for integrated vector management.PLoS Negl Trop Dis. 2025 Jan 27;19(1):e0012795. doi: 10.1371/journal.pntd.0012795. eCollection 2025 Jan. PLoS Negl Trop Dis. 2025. PMID: 39869587 Free PMC article. Review.
References
-
- Maurya, R. P. et al. Biological control: A global perspective. Int. J. Trop. Insect Sci.42, 3203–3220 (2022).10.1007/s42690-022-00881-9 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

