Steatotic liver disease associated with 2,4-dienoyl-CoA reductase 1 deficiency
- PMID: 39277655
- PMCID: PMC11584395
- DOI: 10.1038/s41366-024-01634-z
Steatotic liver disease associated with 2,4-dienoyl-CoA reductase 1 deficiency
Abstract
Background: Metabolic dysfunction-associated steatotic liver disease (MASLD) is considered multifactorial with a number of predisposing gene polymorphisms known.
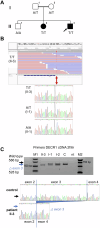
Methods: The occurrence of MASLD in 7 and 10 year old siblings, one without classical risk factors and one with type 2 diabetes suggested a monogenic etiology and prompted next-generation sequencing. Exome sequencing was performed in the proband, both parents and both siblings. The impact of a likely disease-causing DNA variant was assessed on the transcript and protein level.
Results: Two siblings have hepatomegaly, elevated serum transaminase activity, and steatosis and harbor a homozygous DECR1 splice-site variant, c.330+3A>T. The variant caused DECR1 transcript decay. Immunostaining demonstrated lack of DECR1 in patient liver.
Conclusions: These patients may represent the first individuals with DECR1 deficiency, then defining within MASLD an autosomal-recessive entity, well corresponding to the reported steatotic liver disease in Decr1 knockout mice. DECR1 may need to be considered in the genetic work-up of MASLD.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. Human subjects: Genetic studies were approved by the Institutional Review Boards of the Medical University of Innsbruck (No. UN4501), Innsbruck, Austria. The parents provided written informed consent for their participation and that of their children in the study, with clinical data and specimen collection, genetic analysis, and publication of relevant findings.
Figures

References
-
- Younossi ZM, Golabi P, Price JK, Owrangi S, Gundu-Rao N, Satchi R, et al. The global epidemiology of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis among patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2024. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

