Age-related histone H3.3 accumulation associates with a repressive chromatin in mouse tibialis anterior muscle
- PMID: 39277714
- PMCID: PMC11401410
- DOI: 10.1186/s12576-024-00935-2
Age-related histone H3.3 accumulation associates with a repressive chromatin in mouse tibialis anterior muscle
Abstract
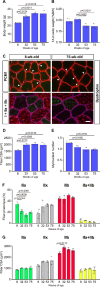
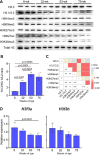
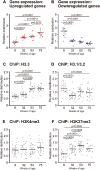
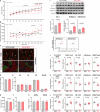
The present study aimed to investigate age-related changes in histone variant H3.3 and its role in the aging process of mouse tibialis anterior muscle. H3.3 level significantly increased with age and correlated with H3K27me3 level. Acute exercise successfully upregulated the target gene expression in 8-wk-old mice, whereas no upregulation was noted in 53-wk-old mice. H3K27me3 level was increased at these loci in response to acute exercise in 8-wk-old mice. However, in 53-wk-old mice, H3.3 and H3K27me3 levels were increased at rest and were not affected by acute exercise. Furthermore, forced H3.3 expression in the skeletal muscle of 8-wk-old mice led to a gradual improvement in motor function. The results suggest that age-related H3.3 accumulation induces the formation of repressive chromatin in the mouse tibialis anterior muscle. However, H3.3 accumulation also appears to play a positive role in enhancing skeletal muscle function.
Keywords: Aging; Chromatin immunoprecipitation; Epigenetics; Exercise; Histone modification.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Exercise-induced histone H3 trimethylation at lysine 27 facilitates the adaptation of skeletal muscle to exercise in mice.J Physiol. 2022 Jul;600(14):3331-3353. doi: 10.1113/JP282917. Epub 2022 Jun 12. J Physiol. 2022. PMID: 35666835
-
Amount of daily exercise is an essential stimulation to alter the epigenome of skeletal muscle in rats.J Appl Physiol (1985). 2018 Oct 1;125(4):1097-1104. doi: 10.1152/japplphysiol.00074.2018. Epub 2018 Aug 2. J Appl Physiol (1985). 2018. PMID: 30070609
-
Chronic exercise training activates histone turnover in mouse skeletal muscle fibers.FASEB J. 2021 Apr;35(4):e21453. doi: 10.1096/fj.202002027RR. FASEB J. 2021. PMID: 33749947
-
A Molecular Prospective for HIRA Complex Assembly and H3.3-Specific Histone Chaperone Function.J Mol Biol. 2017 Jun 30;429(13):1924-1933. doi: 10.1016/j.jmb.2016.11.010. Epub 2016 Nov 19. J Mol Biol. 2017. PMID: 27871933 Free PMC article. Review.
-
The histone variant H3.3 claims its place in the crowded scene of epigenetics.Aging (Albany NY). 2017 Mar 10;9(3):602-614. doi: 10.18632/aging.101194. Aging (Albany NY). 2017. PMID: 28284043 Free PMC article. Review.
Cited by
-
DNA hypermethylation preceded by H3K27 trimethylation is linked to downregulation of gene expression in disuse muscle atrophy in male mice.Physiol Rep. 2025 Apr;13(7):e70317. doi: 10.14814/phy2.70317. Physiol Rep. 2025. PMID: 40223401 Free PMC article.
-
Epigenetic regulation and mechanobiological adaptation in tenocytes during maturation.APL Bioeng. 2025 Jun 26;9(2):026127. doi: 10.1063/5.0271050. eCollection 2025 Jun. APL Bioeng. 2025. PMID: 40584816 Free PMC article.
References
-
- Voisin S, Seale K, Jacques M, Landen S, Harvey NR, Haupt LM, Griffiths LR, Ashton KJ, Coffey VG, Thompson JM, Doering TM, Lindholm ME, Walsh C, Davison G, Irwin R, McBride C, Hansson O, Asplund O, Heikkinen AE, Piirila P, Pietilainen KH, Ollikainen M, Blocquiaux S, Thomis M, Coletta DK, Sharples AP, Eynon N (2024) Exercise is associated with younger methylome and transcriptome profiles in human skeletal muscle. Aging Cell 23:e13859. 10.1111/acel.13859 10.1111/acel.13859 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

