Microbiological and clinical effects of probiotic-related Zeger therapy on gingival health: a randomized controlled clinical trial
- PMID: 39277730
- PMCID: PMC11401283
- DOI: 10.1186/s12903-024-04846-x
Microbiological and clinical effects of probiotic-related Zeger therapy on gingival health: a randomized controlled clinical trial
Abstract
Background: This single-blind randomized controlled trial was aimed to evaluate the microbiological and clinical effects of Zeger therapy on gingival health.
Methods: Twenty-four adults with gingivitis were recruited and monitored micro-biologically and clinically at baseline (Day 0), 4 weeks (Day 29) after therapy. All volunteers received one-stage full-mouth supragingival scaling as basic oral health care for baseline, and then randomly divided into experimental (koumiss, n = 12) or control (none, n = 12) group. The koumiss was used once a day for 4 weeks.
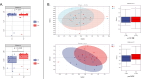
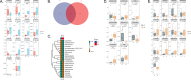
Results: The microbial diversity of the experimental group increased significantly after drinking koumiss (p < 0.05), mainly owing to increasing of Gram-positive bacteria (p = 0.038) and oral health-related microbes (Rothia, Corynebacterium, Actinomyces, Saccharibacteria_TM7, etc.), decreasing of Gram-negative bacteria (p = 0.009) and periodontal disease-related microbes (Porphyromonas, Fusobacterium, Veillonella, etc.), while the microbial diversity of the control group had no significant change (p > 0.05). However, there was no significant difference between the two groups in the clinical parameters (p > 0.05).
Conclusions: Zeger therapy promotes the diversity of supragingival microbiome in adults with gingivitis and increases the abundance of some beneficial flora while decreasing some harmful without clinical parameters marked changing, which holds promise for improving of gingivitis and may be a valuable oral health care approach in the future.
Trial registration: The clinical trial was approved by the Medical Ethics Committee of West China Hospital of Stomatology, Sichuan University, batch No. WCHSIRB-D-2021-428. Before patient registration began, the prospective clinical trial was registered in www.
Clinicaltrials: gov public repository in China under the registration number ChiCTR2200060555 on 04/06/2022.
Keywords: Gingival health; Gingivitis; Oral commensal microbiota; Periodontal disease; Probiotics; Zeger therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Malacarne M, Martuzzi F, Summer A, Mariani P. Protein and fat composition of mare’s milk: some nutritional remarks with reference to human and cow’s milk. Int Dairy J. 2002;12(11):869–77. 10.1016/S0958-6946(02)00120-6 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

