Umbilical cord blood-derived exosomes attenuate dopaminergic neuron damage of Parkinson's disease mouse model
- PMID: 39277761
- PMCID: PMC11401276
- DOI: 10.1186/s12951-024-02773-1
Umbilical cord blood-derived exosomes attenuate dopaminergic neuron damage of Parkinson's disease mouse model
Abstract
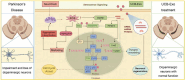
Background: Umbilical cord blood (UCB) is a rich source of multifunctional stem cells characterized by low immunogenicity. Recent research in the fields of aging and regenerative medicine has revealed the potential of human umbilical cord blood-derived exosomes (UCB-Exos) in promoting wound healing, anti-aging, and regeneration. However, their role in neurodegenerative diseases, specifically Parkinson's disease (PD), remains unexplored. This study investigates the potential therapeutic effects and underlying mechanisms of UCB-Exos on PD.
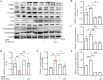
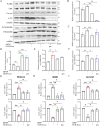
Methods: Large extracellular vesicles (LEv), Exos, and soluble fractions (SF) of human UCB plasma were extracted to investigate their effects on motor dysfunction of the MPTP-induced PD mouse model and identify the key components that improve PD symptoms. UCB-Exos were administered by the caudal vein to prevent or treat the PD mouse model. The motor function and pathological markers were detected. Differentially expressed gene and KEGG enrichment pathways were screened by transcriptome sequence. MN9D and SH-SY5Y cells were cultured and evaluated for cell viability, oxidative stress, cell cycle, and aging-related indexes by qRT-PCR, western blot, immunofluorescence, and flow cytometry. The protein expression level of the MAPK p38 and ERK1/2 signaling pathway was detected by western blot.
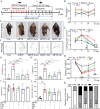
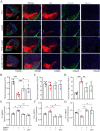
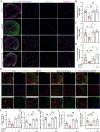
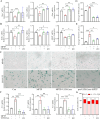
Results: We observed that LEv, Exos, and SF all exhibited potential in ameliorating motor dysfunction in MPTP-induced PD model mice, with UCB-Exos demonstrating the most significant effect. UCB-Exos showed comparable efficacy in preventing and treating motor dysfunction, cognitive decline, and substantia nigra pathological damage in PD mice. Further investigations revealed that UCB-Exos could potentially alleviate oxidative damage, aging and degeneration, and energy metabolism disorders in neurons. Transcriptome sequencing results corroborated that genes differentially expressed due to UCB-Exos were primarily enriched in the neuroactive ligand-receptor interaction, Dopaminergic synapse, and MAPK signaling pathway. We also observed that UCB-Exos significantly inhibited the hyperphosphorylation of the MAPK p38 and ERK1/2 signaling pathways both in vitro and in vivo.
Conclusions: Our study provides a comprehensive evaluation of UCB-Exos on the neuroprotective effects and suggests that inhibition of hyperphosphorylation of MAPK p38 and ERK 1/2 signaling pathways by regulating transcription levels of HspB1 and Ppef2 may be the key mechanism for UCB-Exos to improve PD-related pathological features.
Keywords: Exosome; MAPK signaling pathway; Neuroprotection; Parkinson’s disease; Senescence; Umbilical cord blood.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Iba M, McDevitt RA, Kim C, Roy R, Sarantopoulou D, Tommer E, Siegars B, Sallin M, Kwon S, Sen JM, et al. Aging exacerbates the brain inflammatory micro-environment contributing to alpha-synuclein pathology and functional deficits in a mouse model of DLB/PD. Mol Neurodegener. 2022;17:60. 10.1186/s13024-022-00564-6 - DOI - PMC - PubMed
-
- Schaffner SL, Wassouf Z, Lazaro DF, Xylaki M, Gladish N, Lin DTS, MacIsaac J, Ramadori K, Hentrich T, Schulze-Hentrich JM, et al. Alpha-synuclein overexpression induces epigenomic dysregulation of glutamate signaling and locomotor pathways. Hum Mol Genet. 2022;31:3694–714. 10.1093/hmg/ddac104 - DOI - PMC - PubMed
MeSH terms
Grants and funding
- YC2022003/Innovative research program for graduates of Hubei University of Medicine
- YC2022008/Innovative research program for graduates of Hubei University of Medicine
- YC2023006/Innovative research program for graduates of Hubei University of Medicine
- YC2024017/Innovative research program for graduates of Hubei University of Medicine
- YC2024030/Innovative research program for graduates of Hubei University of Medicine
- WJ2023M161/The Foundation of Health Commission of Hubei Province
- 2024SCOF004/the Open Fund Hubei Provincial Clinical Research Center for Umbilical Cord Blood Hematopoietic Stem Cells, Taihe Hospital
- XYY2023ZY04/Innovative Research Program of Xiangyang No.1 People's Hospital
- 2020DFE025/This work was supported by the Experimental Animal Resources Development and Utilization Project of Hubei Province of China
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

